Switching of INCENP paralogs controls transitions in mitotic chromosomal passenger complex functions
- PMID: 31306061
- PMCID: PMC6681789
- DOI: 10.1080/15384101.2019.1634954
Switching of INCENP paralogs controls transitions in mitotic chromosomal passenger complex functions
Abstract
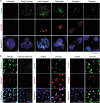
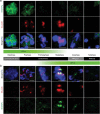
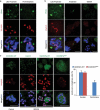
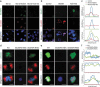
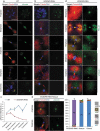
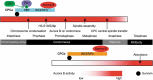
A single inner centromere protein (INCENP) found throughout eukaryotes modulates Aurora B kinase activity and chromosomal passenger complex (CPC) localization, which is essential for timely mitotic progression. It has been proposed that INCENP might act as a rheostat to regulate Aurora B activity through mitosis, with successively higher activity threshold levels for chromosome alignment, the spindle checkpoint, anaphase spindle transfer and finally spindle elongation and cytokinesis. It remains mechanistically unclear how this would be achieved. Here, we reveal that the urochordate, Oikopleura dioica, possesses two INCENP paralogs, which display distinct localizations and subfunctionalization in order to complete M-phase. INCENPa was localized on chromosome arms and centromeres by prometaphase, and modulated Aurora B activity to mediate H3S10/S28 phosphorylation, chromosome condensation, spindle assembly and transfer of the CPC to the central spindle. Polo-like kinase (Plk1) recruitment to CDK1 phosphorylated INCENPa was crucial for INCENPa-Aurora B enrichment on centromeres. The second paralog, INCENPb was enriched on centromeres from prometaphase, and relocated to the central spindle at anaphase onset. In the absence of INCENPa, meiotic spindles failed to form, and homologous chromosomes did not segregate. INCENPb was not required for early to mid M-phase events but became essential for the activity and localization of Aurora B on the central spindle and midbody during cytokinesis in order to allow abscission to occur. Together, our results demonstrate that INCENP paralog switching on centromeres modulates Aurora B kinase localization, thus chronologically regulating CPC functions during fast embryonic divisions in the urochordate O. dioica. Abbreviations: CCAN: constitutive centromere-associated network; CENPs: centromere proteins; cmRNA: capped messenger RNA; CPC: chromosomal passenger complex; INCENP: inner centromere protein; Plk1: polo-like kinase 1; PP1: protein phosphatase 1; PP2A: protein phosphatase 2A; SAC: spindle assembly checkpoint; SAH: single α-helix domain.
Keywords: Aurora B kinase; Inner Centromere Protein (INCENP); abscission; cytokinesis; kinetochore; tunicate.
Figures


Similar articles
-
The chromosomal passenger complex activates Polo kinase at centromeres.PLoS Biol. 2012 Jan;10(1):e1001250. doi: 10.1371/journal.pbio.1001250. Epub 2012 Jan 24. PLoS Biol. 2012. PMID: 22291575 Free PMC article.
-
Centromere localization of INCENP-Aurora B is sufficient to support spindle checkpoint function.Cell Cycle. 2010 Apr 1;9(7):1360-72. doi: 10.4161/cc.9.7.11177. Epub 2010 Apr 1. Cell Cycle. 2010. PMID: 20372054
-
Aurora B kinase activity-dependent and -independent functions of the chromosomal passenger complex in regulating sister chromatid cohesion.J Biol Chem. 2019 Feb 8;294(6):2021-2035. doi: 10.1074/jbc.RA118.005978. Epub 2018 Dec 6. J Biol Chem. 2019. PMID: 30523151 Free PMC article.
-
Role of chromosomal passenger complex in chromosome segregation and cytokinesis.Cell Struct Funct. 2001 Dec;26(6):653-7. doi: 10.1247/csf.26.653. Cell Struct Funct. 2001. PMID: 11942622 Review.
-
Changing places: Chromosomal Passenger Complex relocation in early anaphase.Trends Cell Biol. 2022 Feb;32(2):165-176. doi: 10.1016/j.tcb.2021.09.008. Epub 2021 Oct 16. Trends Cell Biol. 2022. PMID: 34663523 Review.
Cited by
-
Functional specialization of Aurora kinase homologs during oogenic meiosis in the tunicate Oikopleura dioica.Front Cell Dev Biol. 2023 Dec 7;11:1323378. doi: 10.3389/fcell.2023.1323378. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38130951 Free PMC article.
-
Mutation of the SUMOylation site of Aurora-B disrupts spindle formation and chromosome alignment in oocytes.Cell Death Discov. 2024 Oct 22;10(1):447. doi: 10.1038/s41420-024-02217-7. Cell Death Discov. 2024. PMID: 39438456 Free PMC article.
-
A Peak of H3T3 Phosphorylation Occurs in Synchrony with Mitosis in Sea Urchin Early Embryos.Cells. 2020 Apr 7;9(4):898. doi: 10.3390/cells9040898. Cells. 2020. PMID: 32272587 Free PMC article.
-
Recent advances in understanding the role of Cdk1 in the Spindle Assembly Checkpoint.F1000Res. 2020 Jan 28;9:F1000 Faculty Rev-57. doi: 10.12688/f1000research.21185.1. eCollection 2020. F1000Res. 2020. PMID: 32047615 Free PMC article. Review.
-
Aurora kinases signaling in cancer: from molecular perception to targeted therapies.Mol Cancer. 2025 Jun 18;24(1):180. doi: 10.1186/s12943-025-02353-3. Mol Cancer. 2025. PMID: 40533769 Free PMC article. Review.
References
-
- Ruchaud S, Carmena M, Earnshaw WC.. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. - PubMed
-
- Jeyaprakash AA, Klein UR, Lindner D, et al. Structure of a Survivin–borealin–INCENP core complex reveals how chromosomal passengers travel together. Cell. 2007;131:271–285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
