Mechanisms and consequences of gut commensal translocation in chronic diseases
- PMID: 31306081
- PMCID: PMC7053960
- DOI: 10.1080/19490976.2019.1629236
Mechanisms and consequences of gut commensal translocation in chronic diseases
Abstract
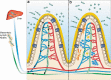
Humans and other mammalian hosts have evolved mechanisms to control the bacteria colonizing their mucosal barriers to prevent invasion. While the breach of barriers by bacteria typically leads to overt infection, increasing evidence supports a role for translocation of commensal bacteria across an impaired gut barrier to extraintestinal sites in the pathogenesis of autoimmune and other chronic, non-infectious diseases. Whether gut commensal translocation is a cause or consequence of the disease is incompletely defined. Here we discuss factors that lead to translocation of live bacteria across the gut barrier. We expand upon our recently published demonstration that translocation of the gut pathobiont Enterococcus gallinarum can induce autoimmunity in susceptible hosts and postulate on the role of Enterococcus species as instigators of chronic, non-infectious diseases.
Keywords: Gut vascular barrier; TLR7; autoimmune liver disease; autoimmunity; bacterial translocation; enterococcus; gut lymphatic barrier; intestinal permeability; lupus; microbiota; tight junctions; vancomycin resistance.
Figures



Similar articles
-
Friend or foe? Lactobacillus in the context of autoimmune disease.Adv Immunol. 2020;146:29-56. doi: 10.1016/bs.ai.2020.02.002. Epub 2020 Mar 19. Adv Immunol. 2020. PMID: 32327152 Review.
-
Within-host evolution of a gut pathobiont facilitates liver translocation.Nature. 2022 Jul;607(7919):563-570. doi: 10.1038/s41586-022-04949-x. Epub 2022 Jul 13. Nature. 2022. PMID: 35831502 Free PMC article.
-
Translocation of a gut pathobiont drives autoimmunity in mice and humans.Science. 2018 Mar 9;359(6380):1156-1161. doi: 10.1126/science.aar7201. Science. 2018. PMID: 29590047 Free PMC article.
-
Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity.Front Immunol. 2021 Apr 22;12:673708. doi: 10.3389/fimmu.2021.673708. eCollection 2021. Front Immunol. 2021. PMID: 33968085 Free PMC article. Review.
-
The microbiome in systemic autoimmune disease: mechanistic insights from recent studies.Curr Opin Rheumatol. 2019 Mar;31(2):201-207. doi: 10.1097/BOR.0000000000000574. Curr Opin Rheumatol. 2019. PMID: 30624285 Free PMC article. Review.
Cited by
-
How Advanced Is Our Understanding of the Role of Intestinal Barrier Dysfunction in the Pathogenesis of Recurrent Urinary Tract Infections.Front Pharmacol. 2022 Mar 10;13:780122. doi: 10.3389/fphar.2022.780122. eCollection 2022. Front Pharmacol. 2022. PMID: 35359839 Free PMC article. Review.
-
Interleukin-17 Weakens the NAFLD/NASH Process by Facilitating Intestinal Barrier Restoration Depending on the Gut Microbiota.mBio. 2022 Apr 26;13(2):e0368821. doi: 10.1128/mbio.03688-21. Epub 2022 Mar 10. mBio. 2022. PMID: 35266816 Free PMC article.
-
The Many Faces of Enterococcus spp.-Commensal, Probiotic and Opportunistic Pathogen.Microorganisms. 2021 Sep 7;9(9):1900. doi: 10.3390/microorganisms9091900. Microorganisms. 2021. PMID: 34576796 Free PMC article. Review.
-
IGA Antibody Induced by Immunization With Pneumococcal Polysaccharides Is a Prognostic Tool in Common Variable Immune Deficiencies.Front Immunol. 2020 Jun 24;11:1283. doi: 10.3389/fimmu.2020.01283. eCollection 2020. Front Immunol. 2020. PMID: 32695106 Free PMC article.
-
Comprehensive insight into the alterations in the gut microbiome and the intestinal barrier as a consequence of iron deficiency anaemia.Biomed J. 2024 Dec;47(6):100701. doi: 10.1016/j.bj.2024.100701. Epub 2024 Jan 26. Biomed J. 2024. PMID: 38281699 Free PMC article.
References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
