Combining antimalarial drugs and vaccine for malaria elimination campaigns: a randomized safety and immunogenicity trial of RTS,S/AS01 administered with dihydroartemisinin, piperaquine, and primaquine in healthy Thai adult volunteers
- PMID: 31306084
- PMCID: PMC7012096
- DOI: 10.1080/21645515.2019.1643675
Combining antimalarial drugs and vaccine for malaria elimination campaigns: a randomized safety and immunogenicity trial of RTS,S/AS01 administered with dihydroartemisinin, piperaquine, and primaquine in healthy Thai adult volunteers
Abstract
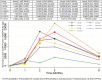
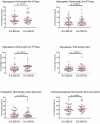
Introduction: RTS,S/AS01 is currently the most advanced malaria vaccine but provides incomplete, short-term protection. It was developed for use within the expanded program on immunizations (EPI) for African children. Another use could be adding mass RTS,S/AS01 vaccination to the integrated malaria elimination strategy in the Greater Mekong Subregion (GMS), where multidrug-resistant P.falciparum strains have emerged and spread. Prior to evaluating RTS,S/AS01 in large-scale trials we assessed whether the vaccine, administered with and without antimalarial drugs, is safe and immunogenic in Asian populations.Methods: An open-label, randomized, controlled phase 2 trial was conducted in healthy, adult Thai volunteers. Seven vaccine regimens with and without antimalarial drugs (dihydroartemisinin-piperaquine plus a single low dose primaquine) were assessed. Antibody titres against the PfCSP full-length (NANP) 6, PfCSP anti-C-term, PfCSP full-length (N + C-Terminal) were measured by standard enzyme-linked immunosorbent assays. Liquid chromatography was used to measure piperaquine, primaquine and carboxy-primaquine concentrations.Results: 193 volunteers were enrolled and 186 study participants completed the 6 months follow-up period. One month after the last vaccination all study participants had seroconverted to the PfCSP (NANP)6, and the PfCSP Full Length (N + C-Terminal). More than 90% had seroconverted to the Pfanti-C-Term CSP. There was no indication that drug concentrations were influenced by vaccine regimens or the antibody levels by the drug regimens. Adverse events were similarly distributed between the seven treatment groups. No serious adverse events attributable to the study interventions were detected.Conclusion: This study found that RTS,S/AS01 with and without dihydroartemisinin-piperaquine plus a single low dose primaquine was safe and immunogenic in a healthy, adult Asian population.
Keywords: ELISA pharmacokinetics; Malaria; P. falciparum; RTS; S/AS01; dihydroartemisinin; phase 2; piperaquine; primaquine; vaccine.
Figures



Similar articles
-
Safety and efficacy of the RTS,S/AS01E candidate malaria vaccine given with expanded-programme-on-immunisation vaccines: 19 month follow-up of a randomised, open-label, phase 2 trial.Lancet Infect Dis. 2011 Oct;11(10):741-9. doi: 10.1016/S1473-3099(11)70100-1. Epub 2011 Jul 22. Lancet Infect Dis. 2011. PMID: 21782519 Clinical Trial.
-
Safety and Immunogenicity of Seven Dosing Regimens of the Candidate RTS,S/AS01E Malaria Vaccine Integrated Within an Expanded Program on Immunization Regimen: A Phase II, Single-Center, Open, Controlled Trial in Infants in Malawi.Pediatr Infect Dis J. 2018 May;37(5):483-491. doi: 10.1097/INF.0000000000001937. Pediatr Infect Dis J. 2018. PMID: 29432383 Clinical Trial.
-
Safety and immunogenicity of the RTS,S/AS01 malaria vaccine in infants and children identified as HIV-infected during a randomized trial in sub-Saharan Africa.Vaccine. 2020 Jan 22;38(4):897-906. doi: 10.1016/j.vaccine.2019.10.077. Epub 2019 Nov 7. Vaccine. 2020. PMID: 31708182 Free PMC article. Clinical Trial.
-
Treatment and prevention of malaria in children.Lancet Child Adolesc Health. 2020 Oct;4(10):775-789. doi: 10.1016/S2352-4642(20)30127-9. Lancet Child Adolesc Health. 2020. PMID: 32946831 Review.
-
[The RTS,S/AS01 malaria vaccine in children aged 5-17 months at first vaccination].Pan Afr Med J. 2018 Jun 19;30:142. doi: 10.11604/pamj.2018.30.142.13152. eCollection 2018. Pan Afr Med J. 2018. PMID: 30374388 Free PMC article. Review. French.
Cited by
-
Vaccine-linked chemotherapy improves cardiac structure and function in a mouse model of chronic Chagas disease.Front Cell Infect Microbiol. 2023 Feb 9;13:1106315. doi: 10.3389/fcimb.2023.1106315. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36844399 Free PMC article.
-
Current Developments in Malaria Vaccination: A Concise Review on Implementation, Challenges, and Future Directions.Clin Pharmacol. 2025 Apr 1;17:29-47. doi: 10.2147/CPAA.S513282. eCollection 2025. Clin Pharmacol. 2025. PMID: 40191019 Free PMC article. Review.
-
ProC6C, a novel multi-stage malaria vaccine, elicits functional antibodies against the minor and central repeats of the Circumsporozoite Protein in human adults.Front Immunol. 2024 Nov 1;15:1481829. doi: 10.3389/fimmu.2024.1481829. eCollection 2024. Front Immunol. 2024. PMID: 39555079 Free PMC article. Clinical Trial.
-
A randomised trial of malaria vaccine R21/Matrix-M™ with and without antimalarial drugs in Thai adults.NPJ Vaccines. 2024 Jul 6;9(1):124. doi: 10.1038/s41541-024-00920-1. NPJ Vaccines. 2024. PMID: 38971837 Free PMC article.
-
Artemisinin resistance and malaria elimination: Where are we now?Front Pharmacol. 2022 Sep 23;13:876282. doi: 10.3389/fphar.2022.876282. eCollection 2022. Front Pharmacol. 2022. PMID: 36210819 Free PMC article. Review.
References
-
- RTSS._Clinical_Trials_Partnership, Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmüller B, Issifou S, Kremsner PG, et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012;367:2284–95. doi:10.1056/NEJMoa1208394. - DOI - PMC - PubMed
-
- RTSS._Clinical_Trials_Partnership, Agnandji ST, Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BGNO, Doucka Y, Flamen A, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365:1863–75. doi:10.1056/NEJMoa1102287. - DOI - PubMed
-
- RTSS_Clinical_Trials_Partnership . Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi:10.1016/S0140-6736(15)60721-8. - DOI - PMC - PubMed
-
- Regules JA, Cicatelli SB, Bennett JW, Paolino KM, Twomey PS, Moon JE, Kathcart AK, Hauns KD, Komisar JL, Qabar AN, et al. Fractional third and fourth dose of RTS,S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J Infect Dis. 2016;214:762–71. doi:10.1093/infdis/jiw237. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
