Endothelial Mitochondrial Dysfunction in Cerebral Amyloid Angiopathy and Alzheimer's Disease
- PMID: 31306129
- PMCID: PMC6917858
- DOI: 10.3233/JAD-190357
Endothelial Mitochondrial Dysfunction in Cerebral Amyloid Angiopathy and Alzheimer's Disease
Abstract
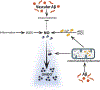
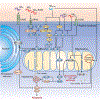
Alzheimer's disease (AD) is the most prevalent form of dementia. Cerebrovascular dysfunction is one of the earliest events in the pathogenesis of AD, as well as in vascular and mixed dementias. Cerebral amyloid angiopathy (CAA), the deposition of amyloid around cerebral vessels, is observed in up to 90% of AD patients and in approximately 50% of elderly individuals over 80 years of age. CAA is a strong contributor to vascular dysfunction in AD. CAA-laden brain vessels are characterized by dysfunctional hemodynamics and leaky blood-brain barrier (BBB), contributing to clearance failure and further accumulation of amyloid-β (Aβ) in the cerebrovasculature and brain parenchyma. Mitochondrial dysfunction is increasingly recognized as an important early initiator of the pathogenesis of AD and CAA. The objective of this review is to discuss the effects of Aβ on cerebral microvascular cell function, focusing on its impact on endothelial mitochondria. After introducing CAA and its etiology and genetic risk factors, we describe the pathological relationship between cerebrovascular amyloidosis and brain microvascular endothelial cell dysfunction, critically analyzing its roles in disease progression, hypoperfusion, and BBB integrity. Then, we focus on discussing the effect of Aβ challenge on endothelial mitochondrial dysfunction pathways, and their contribution to the progression of neurovascular dysfunction in AD and dementia. Finally, we report potential pharmacological and non-pharmacological mitochondria-targeted therapeutic strategies which may help prevent or delay cerebrovascular failure.
Keywords: Alzheimer’s disease; amyloid; apoptosis; blood-brain barrier; cerebral amyloid angiopathy; endothelial cells; mitochondria; neurodegeneration; reactive nitrogen species; reactive oxygen species.
Figures
References
-
- Alzheimer’s Association (2019) 2019 Alzheimer’s disease facts and figures. Alzheimers Dement 15, 321–387.
-
- Corriveau RA, Bosetti F, Emr M, Gladman JT, Koenig JI, Moy CS, Pahigiannis K, Waddy SP, Koroshetz W (2016) The science of vascular contributions to cognitive impairment and dementia (VCID): A framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell Mol Neurobiol 36, 281–288. - PMC - PubMed
-
- Jellinger KA, Attems J (2005) Prevalence and pathogenic role of cerebrovascular lesions in Alzheimer disease. J Neurol Sci 229–230, 37–41. - PubMed
-
- Jellinger KA, Attems J (2007) Neuropathological evaluation of mixed dementia. J Neurol Sci 257, 80–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

