The Qualification of an Enrichment Biomarker for Clinical Trials Targeting Early Stages of Parkinson's Disease
- PMID: 31306141
- PMCID: PMC6700608
- DOI: 10.3233/JPD-191648
The Qualification of an Enrichment Biomarker for Clinical Trials Targeting Early Stages of Parkinson's Disease
Erratum in
-
The Qualification of an Enrichment Biomarker for Clinical Trials Targeting Early Stages of Parkinson's Disease.J Parkinsons Dis. 2019;9(4):825. doi: 10.3233/JPD-199003. J Parkinsons Dis. 2019. PMID: 31524182 Free PMC article. No abstract available.
Abstract
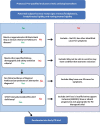
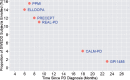
As therapeutic trials target early stages of Parkinson's disease (PD), appropriate patient selection based purely on clinical criteria poses significant challenges. Members of the Critical Path for Parkinson's Consortium formally submitted documentation to the European Medicines Agency (EMA) supporting the use of Dopamine Transporter (DAT) neuroimaging in early PD. Regulatory documents included a comprehensive literature review, a proposed analysis plan of both observational and clinical trial data, and an assessment of biomarker reproducibility and reliability. The research plan included longitudinal analysis of the Parkinson Research Examination of CEP-1347 Trial (PRECEPT) and the Parkinson's Progression Markers Initiative (PPMI) study to estimate the degree of enrichment achieved and impact on future trials in subjects with early motor PD. The presence of reduced striatal DAT binding based on visual reads of single photon emission tomography (SPECT) scans in early motor PD subjects was an independent predictor of faster decline in UPDRS Parts II and III as compared to subjects with scans without evidence of dopaminergic deficit (SWEDD) over 24 months. The EMA issued in 2018 a full Qualification Opinion for the use of DAT as an enrichment biomarker in PD trials targeting subjects with early motor symptoms. Exclusion of SWEDD subjects in future clinical trials targeting early motor PD subjects aims to enrich clinical trial populations with idiopathic PD patients, improve statistical power, and exclude subjects who are unlikely to progress clinically from being exposed to novel test therapeutics.
Keywords: Dopamine transporter; EMA; PPMI; PRECEPT; SWEDD; enrichment biomarker.
Conflict of interest statement
Authors who are employed at pharmaceutical or diagnostic companies have respective conflicts of interest based on their affiliations.
MT, SV, DS, KR, DC, AR, JG, DD, SI have no conflicts of interest.
JS: Consultant (Biogen, Roche, LikeMinds, Life Molecular Imaging); Equity (Invicro).
DG: received grant funding from the Neurosciences Foundation, Michael’s Movers, and Parkinson’s UK, and honoraria from Bial Pharmaceuticals and GE Healthcare.
GS: received grant funding from NIH, Michael J Fox Foundation and honoraria from Biogen.
KM: received funding from the The Michael J. Fox Foundation, the US Department of Defense and is employed by Invicro and has received consultant fees from Pfizer, GE Healthcare, Lilly, BMS, Piramal, Biogen, Prothena, Roche, Neuropore,US Worldmeds, Neurophage, UCB, Oxford Biomedica, Lysosomal Therapetic, Inc, Neuroderm, Denali and the Michael J. Fox Foundation.
Figures
Similar articles
-
Longitudinal follow-up of SWEDD subjects in the PRECEPT Study.Neurology. 2014 May 20;82(20):1791-7. doi: 10.1212/WNL.0000000000000424. Epub 2014 Apr 23. Neurology. 2014. PMID: 24759846 Free PMC article.
-
Dopamine Transporter Neuroimaging as an Enrichment Biomarker in Early Parkinson's Disease Clinical Trials: A Disease Progression Modeling Analysis.Clin Transl Sci. 2018 Jan;11(1):63-70. doi: 10.1111/cts.12492. Epub 2017 Jul 27. Clin Transl Sci. 2018. PMID: 28749580 Free PMC article.
-
Value of Clinical Signs in Identifying Patients with Scans without Evidence of Dopaminergic Deficit (SWEDD).J Parkinsons Dis. 2020;10(4):1561-1569. doi: 10.3233/JPD-202090. J Parkinsons Dis. 2020. PMID: 32597819 Free PMC article. Clinical Trial.
-
Dopamine transporter SPECT imaging in Parkinson’s disease and parkinsonian disorders.Turk J Med Sci. 2021 Apr 30;51(2):400-410. doi: 10.3906/sag-2008-253. Turk J Med Sci. 2021. PMID: 33237660 Free PMC article. Review.
-
The role of SPECT imaging of the dopaminergic system in translational research on Parkinson's disease.Parkinsonism Relat Disord. 2014 Jan;20 Suppl 1:S184-6. doi: 10.1016/S1353-8020(13)70043-9. Parkinsonism Relat Disord. 2014. PMID: 24262177 Review.
Cited by
-
Simplified quantification of [18F]FE-PE2I PET in Parkinson's disease: Discriminative power, test-retest reliability and longitudinal validity during early peak and late pseudo-equilibrium.J Cereb Blood Flow Metab. 2021 Jun;41(6):1291-1300. doi: 10.1177/0271678X20958755. Epub 2020 Sep 21. J Cereb Blood Flow Metab. 2021. PMID: 32955955 Free PMC article. Clinical Trial.
-
Approaches to Disease Modification for Parkinson's Disease: Clinical Trials and Lessons Learned.Neurotherapeutics. 2020 Oct;17(4):1393-1405. doi: 10.1007/s13311-020-00964-w. Epub 2020 Nov 17. Neurotherapeutics. 2020. PMID: 33205384 Free PMC article. Review.
-
Precompetitive Consensus Building to Facilitate the Use of Digital Health Technologies to Support Parkinson Disease Drug Development through Regulatory Science.Digit Biomark. 2020 Nov 26;4(Suppl 1):28-49. doi: 10.1159/000512500. eCollection 2020 Winter. Digit Biomark. 2020. PMID: 33442579 Free PMC article. Review.
-
Open Data Revolution in Clinical Research: Opportunities and Challenges.Clin Transl Sci. 2020 Jul;13(4):665-674. doi: 10.1111/cts.12756. Epub 2020 Mar 10. Clin Transl Sci. 2020. PMID: 32004409 Free PMC article. Review.
-
Neuroimaging Biomarkers in Parkinson's Disease.Adv Neurobiol. 2024;40:617-663. doi: 10.1007/978-3-031-69491-2_21. Adv Neurobiol. 2024. PMID: 39562459 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

