Novel Methylated DNA Markers Discriminate Advanced Neoplasia in Pancreatic Cysts: Marker Discovery, Tissue Validation, and Cyst Fluid Testing
- PMID: 31306149
- PMCID: PMC7294458
- DOI: 10.14309/ajg.0000000000000284
Novel Methylated DNA Markers Discriminate Advanced Neoplasia in Pancreatic Cysts: Marker Discovery, Tissue Validation, and Cyst Fluid Testing
Abstract
Objectives: Pancreatic cystic lesions (PCLs) may be precancerous. Those likely to harbor high-grade dysplasia (HGD) or pancreatic cancer (PC) are targets for surgical resection. Current algorithms to predict advanced neoplasia (HGD/PC) in PCLs lack diagnostic accuracy. In pancreatic tissue and cyst fluid (CF) from PCLs, we sought to identify and validate novel methylated DNA markers (MDMs) that discriminate HGD/PC from low-grade dysplasia (LGD) or no dysplasia (ND).
Methods: From an unbiased whole-methylome discovery approach using predefined selection criteria followed by multistep validation on case (HGD or PC) and control (ND or LGD) tissues, we identified discriminant MDMs. Top candidate MDMs were then assayed by quantitative methylation-specific polymerase chain reaction on archival CF from surgically resected PCLs.
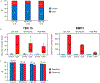
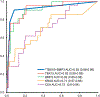
Results: Of 25 discriminant MDMs identified in tissue, 13 were selected for validation in 134 CF samples (21 cases [8 HGD, 13 PC], 113 controls [45 ND, 68 LGD]). A tree-based algorithm using 2 CF-MDMs (TBX15, BMP3) achieved sensitivity and specificity above 90%. Discrimination was significantly better by this CF-MDM panel than by mutant KRAS or carcinoembryonic antigen, with areas under the receiver operating characteristic curve of 0.93 (95% confidence interval: 0.86-0.99), 0.71 (0.57-0.85), and 0.72 (0.60-0.84), respectively. Cutoffs for the MDM panel applied to an independent CF validation set (31 cases, 56 controls) yielded similarly high discrimination, areas under the receiver operating characteristic curve = 0.86 (95% confidence interval: 0.77-0.94, P = 0.2).
Discussion: Novel MDMs discovered and validated in tissue accurately identify PCLs harboring HGD/PC. A panel of 2 MDMs assayed in CF yielded results with potential to enhance current risk prediction algorithms. Prospective studies are indicated to optimize and further evaluate CF-MDMs for clinical use.
Conflict of interest statement
CONFLICTS OF INTEREST
Figures




References
-
- Moris M, Bridges MD, Pooley RA, et al. Association between advances in high-resolution cross-section imaging technologies and increase in prevalence of pancreatic cysts from 2005 to 2014. Clin Gastroenterol Hepatol 2016;14:585–93.e3. - PubMed
-
- Lee KS, Sekhar A, Rofsky NM, et al. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 2010; 105:2079–84. - PubMed
-
- de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol 2010;8:806–11. - PubMed
-
- Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015;148: 824–48.e22. - PubMed
-
- Tada M, Kawabe T, Arizumi M, et al. Pancreatic cancer in patients with pancreatic cystic lesions: A prospective study in 197 patients. Clin Gastroenterol Hepatol 2006;4:1265–70. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

