Suboptimal stimulation by weak agonist epitope variants does not drive dysfunction of HIV-1-specific cytotoxic T lymphocyte clones
- PMID: 31306165
- PMCID: PMC7046083
- DOI: 10.1097/QAD.0000000000002259
Suboptimal stimulation by weak agonist epitope variants does not drive dysfunction of HIV-1-specific cytotoxic T lymphocyte clones
Abstract
Objective: To assess whether weakly recognized epitope variants induce anergy in HIV-1-specific CD8 T lymphocyte (CTL) clones as a mechanism of dysfunction.
Design: HIV-1-specific CTL clones were exposed to suboptimally recognized epitope variants, and screened for anergy and other T-cell dysfunction markers, and subsequent capability to kill target cells bearing index epitope.
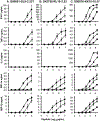
Methods: In addition to the optimally recognized index epitope, two suboptimally recognized epitope variants were selected based on titration curves for killing of peptide-labeled target cells by three HIV-1-specific CTL clones targeting the epitopes SLYNTVATL (Gag 77-85, A02-restricted), RPAEPVPLQL (Rev 66-75, B07-restricted), and KRWIIMGLNK (Gag 263-272, B27-restricted). Consequences of suboptimal stimulation were assessed by cytokine secretion, gene expression, and capacity to kill index epitope-labeled target cells upon rechallenge.
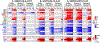
Results: Suboptimal recognition of epitope variants reduced cytokine production by CTL similarly to reduction in killing of target cells. Gene expression profiles after suboptimal stimulation demonstrated no patterns consistent with T-cell dysfunction due to anergy, exhaustion, or apoptosis. Preexposure of CTL to epitope variants had no discernable impact on their subsequent capacity to kill index epitope-bearing target cells.
Conclusion: Our data explore the hypothesis that poorly recognized epitope variants not only facilitate HIV-1 evasion of CTL recognition, but also induce CTL dysfunction through suboptimal signaling causing anergy. However, the results do not suggest that suboptimal signaling induces anergy (or exhaustion or apoptosis), indicating that the major role of CTL epitope variation is likely viral escape.
Conflict of interest statement
Conflicts of Interest and Sources of Funding: O.O.Y. serves as Director for Scientific Research at AIDS Healthcare Foundation; for the remaining authors no conflicts of interest are declared.
Figures
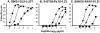


Similar articles
-
Recognition patterns of HLA-A2-restricted human immunodeficiency virus-1-specific cytotoxic T-lymphocytes in a cohort of HIV-1-infected individuals.Viral Immunol. 2005;18(4):627-36. doi: 10.1089/vim.2005.18.627. Viral Immunol. 2005. PMID: 16359229
-
Impaired processing and presentation of cytotoxic-T-lymphocyte (CTL) epitopes are major escape mechanisms from CTL immune pressure in human immunodeficiency virus type 1 infection.J Virol. 2004 Feb;78(3):1324-32. doi: 10.1128/jvi.78.3.1324-1332.2004. J Virol. 2004. PMID: 14722287 Free PMC article.
-
The HIV-1 HLA-A2-SLYNTVATL is a help-independent CTL epitope.J Immunol. 2004 May 1;172(9):5249-61. doi: 10.4049/jimmunol.172.9.5249. J Immunol. 2004. PMID: 15100263
-
Efficient processing of the immunodominant, HLA-A*0201-restricted human immunodeficiency virus type 1 cytotoxic T-lymphocyte epitope despite multiple variations in the epitope flanking sequences.J Virol. 1999 Dec;73(12):10191-8. doi: 10.1128/JVI.73.12.10191-10198.1999. J Virol. 1999. PMID: 10559335 Free PMC article.
-
Human immunodeficiency virus type 1-specific cytotoxic T lymphocytes release gamma interferon, tumor necrosis factor alpha (TNF-alpha), and TNF-beta when they encounter their target antigens.J Virol. 1993 May;67(5):2844-52. doi: 10.1128/JVI.67.5.2844-2852.1993. J Virol. 1993. PMID: 7682629 Free PMC article.
Cited by
-
Non-synonymous Substitutions in HIV-1 GAG Are Frequent in Epitopes Outside the Functionally Conserved Regions and Associated With Subtype Differences.Front Microbiol. 2021 Jan 11;11:615721. doi: 10.3389/fmicb.2020.615721. eCollection 2020. Front Microbiol. 2021. PMID: 33505382 Free PMC article.
-
Recent Advances and the Mechanism of Astaxanthin in Ophthalmological Diseases.J Ophthalmol. 2022 May 20;2022:8071406. doi: 10.1155/2022/8071406. eCollection 2022. J Ophthalmol. 2022. PMID: 35646393 Free PMC article. Review.
References
-
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006,443:350–354. - PubMed
-
- Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, Meyers H, et al. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med 1997,3:205–211. - PubMed
-
- Phillips RE, Rowland-Jones S, Nixon DF, Gotch FM, Edwards JP, Ogunlesi AO, et al. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature 1991,354:453–459. - PubMed

