Effect of OTX-101, a Novel Nanomicellar Formulation of Cyclosporine A, on Corneal Staining in Patients With Keratoconjunctivitis Sicca: A Pooled Analysis of Phase 2b/3 and Phase 3 Studies
- PMID: 31306284
- PMCID: PMC6749965
- DOI: 10.1097/ICO.0000000000001989
Effect of OTX-101, a Novel Nanomicellar Formulation of Cyclosporine A, on Corneal Staining in Patients With Keratoconjunctivitis Sicca: A Pooled Analysis of Phase 2b/3 and Phase 3 Studies
Erratum in
-
Effect of OTX-101, a Novel Nanomicellar Formulation of Cyclosporine A, on Corneal Staining in Patients With Keratoconjunctivitis Sicca: A Pooled Analysis of Phase 2b/3 and Phase 3 Studies: Erratum.Cornea. 2020 Feb;39(2):e10. doi: 10.1097/ICO.0000000000002221. Cornea. 2020. PMID: 31714407 Free PMC article. No abstract available.
Abstract
Background: Keratoconjunctivitis sicca affects 5% to 33% of the population and is often accompanied by symptoms such as burning and dryness. This pooled analysis evaluated total and central corneal fluorescein staining (CFS) in patients receiving OTX-101 0.09% or vehicle in phase 2b/3 and 3 studies and whether improvements in corneal staining correlated with improved visual acuity.
Methods: In these randomized, vehicle-controlled studies, patients received 1 drop of OTX-101 or vehicle in both eyes twice daily. Corneal staining was performed at baseline and days 28, 56, and 84. CFS was evaluated in each zone (0-to-4 scale); total corneal staining (0-to-20 scale per eye) was averaged over both eyes. Pooled safety assessments included adverse event monitoring.
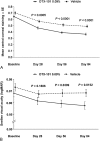
Results: Mean baseline CFS total scores (SD) were 4.2 (2.5) and 4.3 (2.6) for the OTX-101 (n = 523) and vehicle (n = 525) groups, respectively. For total corneal staining, least squares mean changes from baseline (standard error) were -0.9 (0.08) versus -0.5 (0.08) for OTX-101 and vehicle, respectively (P = 0.0008), on day 28 and -1.4 (0.09) versus -0.9 (0.09) on day 84 (P = 0.0002). There was a significantly high correlation (P = 0.0117) between reduced central corneal staining and improved visual acuity on day 84. Treatment-related adverse events were mostly mild, with instillation site pain reported by 21.8% and 4.0% of patients receiving OTX-101 and vehicle, respectively.
Conclusions: Treatment with OTX-101 led to greater improvements versus vehicle in corneal surface staining as early as 4 weeks, and further improvements were seen up to 12 weeks. OTX-101 was well tolerated in patients with keratoconjunctivitis sicca.
Figures



References
-
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–283. - PubMed
-
- Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438–510. - PubMed
-
- Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15:539–574. - PubMed
-
- Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15:334–365. - PubMed
-
- Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15:575–628. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

