Persistent Digermenes with Acyl and α-Chlorosilyl Functionalities
- PMID: 31306508
- PMCID: PMC6852044
- DOI: 10.1002/chem.201902553
Persistent Digermenes with Acyl and α-Chlorosilyl Functionalities
Abstract
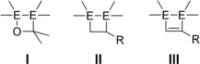
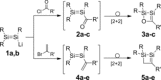
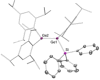
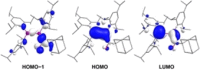
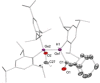
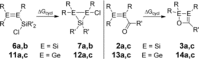
We report the preparation of α-chlorosilyl- and acyl-substituted digermenes. Unlike the corresponding transient disilenes, these species with a Ge=Ge double bond show an unexpectedly low tendency for cyclization, but in turn are prone to thermal Ge=Ge bond cleavage. Triphenylsilyldigermene has been isolated as a crystalline model compound, and is the first fully characterized example of a neutral digermene with an A2 GeGeAB substitution pattern. Spectroscopic and computational evidence prove the constitution of 1-adamantoyldigermene as a first persistent species with a heavy double bond conjugated with a carbonyl moiety.
Keywords: cycloaddition; digermenes; double bonds; germanium; isomerization.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
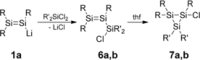


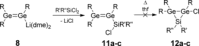

Similar articles
-
The addition of nitriles to a molecular digermene: reversible addition and comparison to surface reactivity.Angew Chem Int Ed Engl. 2015 May 26;54(22):6600-3. doi: 10.1002/anie.201501278. Epub 2015 Apr 13. Angew Chem Int Ed Engl. 2015. PMID: 25864389
-
A multiply functionalized base-coordinated Ge(II) compound and its reversible dimerization to the digermene.Angew Chem Int Ed Engl. 2015 Jan 2;54(1):289-92. doi: 10.1002/anie.201407751. Epub 2014 Oct 31. Angew Chem Int Ed Engl. 2015. PMID: 25363405
-
Addition of Isocyanides to Tetramesityldigermene: A Comparison of the Reactivity between Surface and Molecular Digermenes.Chemistry. 2016 Sep 19;22(39):14006-14012. doi: 10.1002/chem.201602222. Epub 2016 Aug 16. Chemistry. 2016. PMID: 27529452
-
Spontaneous Decomposition of an Extraordinarily Twisted and Trans-Bent Fully-Phosphanyl-Substituted Digermene to an Unusual GeI Cluster.Angew Chem Int Ed Engl. 2022 Sep 26;61(39):e202208851. doi: 10.1002/anie.202208851. Epub 2022 Aug 25. Angew Chem Int Ed Engl. 2022. PMID: 35946808 Free PMC article.
-
Nephrotoxicity and neurotoxicity in humans from organogermanium compounds and germanium dioxide.Biol Trace Elem Res. 1991 Jun;29(3):267-80. doi: 10.1007/BF03032683. Biol Trace Elem Res. 1991. PMID: 1726409 Review.
Cited by
-
Organogermanium Analogues of Alkenes, Alkynes, 1,3-Dienes, Allenes, and Vinylidenes.Molecules. 2023 Feb 6;28(4):1558. doi: 10.3390/molecules28041558. Molecules. 2023. PMID: 36838546 Free PMC article. Review.
-
A Mixed Heavier Si=Ge Analogue of a Vinyl Anion.Angew Chem Int Ed Engl. 2021 Jan 4;60(1):242-246. doi: 10.1002/anie.202009406. Epub 2020 Oct 26. Angew Chem Int Ed Engl. 2021. PMID: 32991043 Free PMC article.
-
N-Heterocyclic Carbene Stabilized Bisacylgermylenes.Chemistry. 2025 Jul 8;31(38):e202501707. doi: 10.1002/chem.202501707. Epub 2025 Jun 12. Chemistry. 2025. PMID: 40464281 Free PMC article.
References
-
- Goldberg D. E., Harris D. H., Lappert M. F., Thomas K. M., J. Chem. Soc. Chem. Commun. 1976, 227, 261.
-
- None
-
- West R., Fink M. J., Science 1981, 214, 1343–1344; - PubMed
-
- Hitchcock P. B., Lappert M. F., Miles S. J., Thorne A. J., J. Chem. Soc. Chem. Commun. 1984, 480–482;
-
- Stender M., Phillips A. D., Wright R. J., Power P. P., Angew. Chem. Int. Ed. 2002, 41, 1785–1787; - PubMed
- Angew. Chem. 2002, 114, 1863–1865;
Grants and funding
LinkOut - more resources
Full Text Sources

