Characterizing the inhibition of α-synuclein oligomerization by a pharmacological chaperone that prevents prion formation by the protein PrP
- PMID: 31306510
- PMCID: PMC6699102
- DOI: 10.1002/pro.3684
Characterizing the inhibition of α-synuclein oligomerization by a pharmacological chaperone that prevents prion formation by the protein PrP
Abstract
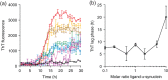
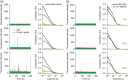
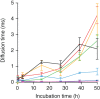
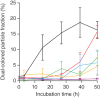
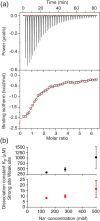
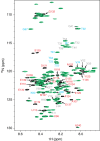
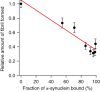
Aggregation of the disordered protein α-synuclein into amyloid fibrils is a central feature of synucleinopathies, neurodegenerative disorders that include Parkinson's disease. Small, pre-fibrillar oligomers of misfolded α-synuclein are thought to be the key toxic entities, and α-synuclein misfolding can propagate in a prion-like way. We explored whether a compound with anti-prion activity that can bind to unfolded parts of the protein PrP, the cyclic tetrapyrrole Fe-TMPyP, was also active against α-synuclein aggregation. Observing the initial stages of aggregation via fluorescence cross-correlation spectroscopy, we found that Fe-TMPyP inhibited small oligomer formation in a dose-dependent manner. Fe-TMPyP also inhibited the formation of mature amyloid fibrils in vitro, as detected by thioflavin T fluorescence. Isothermal titration calorimetry indicated Fe-TMPyP bound to monomeric α-synuclein with a stoichiometry of 2, and two-dimensional heteronuclear single quantum coherence NMR spectra revealed significant interactions between Fe-TMPyP and the C-terminus of the protein. These results suggest commonalities among aggregation mechanisms for α-synuclein and the prion protein may exist that can be exploited as therapeutic targets.
Keywords: cyclic tetrapyrrole; fluorescence cross-correlation spectroscopy; isothermal titration calorimetry; protein aggregation.
© 2019 The Protein Society.
Conflict of interest statement
All authors declare that they have no conflict of interest with the contents of this publication.
Figures
Similar articles
-
Clustering of human prion protein and α-synuclein oligomers requires the prion protein N-terminus.Commun Biol. 2020 Jul 9;3(1):365. doi: 10.1038/s42003-020-1085-z. Commun Biol. 2020. PMID: 32647130 Free PMC article.
-
A cationic tetrapyrrole inhibits toxic activities of the cellular prion protein.Sci Rep. 2016 Mar 15;6:23180. doi: 10.1038/srep23180. Sci Rep. 2016. PMID: 26976106 Free PMC article.
-
Pathogenic mechanisms of prion protein, amyloid-β and α-synuclein misfolding: the prion concept and neurotoxicity of protein oligomers.J Neurochem. 2016 Oct;139(2):162-180. doi: 10.1111/jnc.13772. Epub 2016 Sep 15. J Neurochem. 2016. PMID: 27529376 Review.
-
The role of the prion protein in the internalization of α-synuclein amyloids.Prion. 2018 Jan 2;12(1):23-27. doi: 10.1080/19336896.2017.1423186. Epub 2018 Jan 31. Prion. 2018. PMID: 29308725 Free PMC article.
-
Structures of Oligomeric States of Tau Protein, Amyloid-β, α-Synuclein and Prion Protein Implicated in Alzheimer's Disease, Parkinson's Disease and Prionopathies.Int J Mol Sci. 2024 Dec 4;25(23):13049. doi: 10.3390/ijms252313049. Int J Mol Sci. 2024. PMID: 39684761 Free PMC article. Review.
Cited by
-
Direct Cytosolic Delivery of Proteins Using Lyophilized and Reconstituted Polymer-Protein Assemblies.Pharm Res. 2022 Jun;39(6):1197-1204. doi: 10.1007/s11095-022-03226-w. Epub 2022 Mar 16. Pharm Res. 2022. PMID: 35297498 Free PMC article.
-
Bivalent metal ions induce formation of α-synuclein fibril polymorphs with different cytotoxicities.Sci Rep. 2022 Jul 13;12(1):11898. doi: 10.1038/s41598-022-15472-4. Sci Rep. 2022. PMID: 35831343 Free PMC article.
-
Polymorphic Alpha-Synuclein Oligomers: Characterization and Differential Detection with Novel Corresponding Antibodies.Mol Neurobiol. 2023 May;60(5):2691-2705. doi: 10.1007/s12035-023-03211-3. Epub 2023 Jan 28. Mol Neurobiol. 2023. PMID: 36707462 Free PMC article.
-
A native chemical chaperone in the human eye lens.Elife. 2022 Jun 20;11:e76923. doi: 10.7554/eLife.76923. Elife. 2022. PMID: 35723573 Free PMC article.
-
Acute Intermittent Porphyria: An Overview of Therapy Developments and Future Perspectives Focusing on Stabilisation of HMBS and Proteostasis Regulators.Int J Mol Sci. 2021 Jan 12;22(2):675. doi: 10.3390/ijms22020675. Int J Mol Sci. 2021. PMID: 33445488 Free PMC article. Review.
References
-
- Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT Jr. NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. - PubMed
-
- Bertoncini CW, Fernandez CO, Griesinger C, Jovin TM, Zweckstetter M. Familial mutants of alpha‐synuclein with increased neurotoxicity have a destabilized conformation. J Biol Chem. 2005;280:30649–30652. - PubMed
-
- Iwai A, Masliah E, Yoshimoto M, et al. The precursor protein of non‐A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. - PubMed
-
- Wood SJ, Wypych J, Steavenson S, Louis JC, Citron M, Biere AL. Alpha‐Synuclein fibrillogenesis is nucleation‐dependent implications for the pathogenesis of Parkinson's disease. J Biol Chem. 1999;274:19509–19512. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

