Long-term safety and efficacy of trifarotene 50 μg/g cream, a first-in-class RAR-γ selective topical retinoid, in patients with moderate facial and truncal acne
- PMID: 31306527
- PMCID: PMC7004112
- DOI: 10.1111/jdv.15794
Long-term safety and efficacy of trifarotene 50 μg/g cream, a first-in-class RAR-γ selective topical retinoid, in patients with moderate facial and truncal acne
Abstract
Background: Treatment for both facial and truncal acne has not sufficiently been studied.
Objectives: To evaluate the long-term safety and efficacy of trifarotene in both facial and truncal acne.
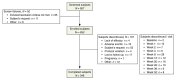
Methods: In a multicentre, open-label, 52-week study, patients with moderate facial and truncal acne received trifarotene 50 μg/g cream (trifarotene). Assessments included local tolerability, safety, investigator and physician's global assessments (IGA, PGA) and quality of life (QOL). A validated QOL questionnaire was completed by the patient at Baseline, Week 12, 26 and 52/ET.
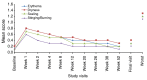
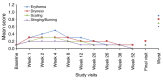
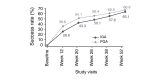
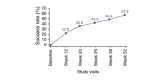
Results: Of 453 patients enrolled, 342 (75.5%) completed the study. Trifarotene-related treatment-emergent adverse events (TEAEs) were reported in 12.6% of patients, and none was serious. Most related TEAEs were cutaneous and occurred during the first 3 months. Signs and symptoms of local tolerability were mostly mild or moderate and severe signs, and symptoms were reported for 2.2% to 7.1% of patients for the face and 2.5% to 5.4% for the trunk. Local irritation increased during the first week of treatment on the face and up to Weeks 2 to 4 on the trunk with both decreasing thereafter. At Week 12, IGA and PGA success rates were 26.6% and 38.6%, respectively. Success rates increased to 65.1% and 66.9%, respectively at Week 52. Overall success (both IGA and PGA success in the same patient) was 57.9% at Week 52. At Week 52 visit, 92/171 (53.8%) patients who had completed their assessments had scores from 0 to 1 (i.e. no effect of acne on their QOL) vs. 47/208 (22.6%) patients at Baseline visit.
Conclusion: In this 52-week study, trifarotene was safe, well tolerated and effective in moderate facial and truncal acne.
© 2019 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures

Similar articles
-
Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne.J Am Acad Dermatol. 2019 Jun;80(6):1691-1699. doi: 10.1016/j.jaad.2019.02.044. Epub 2019 Feb 22. J Am Acad Dermatol. 2019. PMID: 30802558 Clinical Trial.
-
Trifarotene for the Treatment of Facial and Truncal Acne.Ann Pharmacother. 2021 Jan;55(1):111-116. doi: 10.1177/1060028020934892. Epub 2020 Jun 21. Ann Pharmacother. 2021. PMID: 32567361
-
A Novel Topical Retinoid for Acne: Trifarotene 50 μg/g Cream.Skin Therapy Lett. 2020 Mar;25(2):1-2. Skin Therapy Lett. 2020. PMID: 32196146
-
Management of Acne Vulgaris With Trifarotene.J Cutan Med Surg. 2023 Jul-Aug;27(4):368-374. doi: 10.1177/12034754231163542. Epub 2023 Mar 16. J Cutan Med Surg. 2023. PMID: 36927117 Free PMC article. Review.
-
Advances in Topical Management of Adolescent Facial and Truncal Acne: A Phase 3 Pooled Analysis of Safety and Efficacy of Trifarotene 0.005% Cream.J Drugs Dermatol. 2022 Jun 1;21(6):582-586. doi: 10.36849/JDD.6778. J Drugs Dermatol. 2022. PMID: 35674762
Cited by
-
Trifarotene 0.005% Cream in the Treatment of Facial and Truncal Acne Vulgaris in Patients with Skin of Color: a Case Series.Dermatol Ther (Heidelb). 2022 Sep;12(9):2189-2200. doi: 10.1007/s13555-022-00788-w. Epub 2022 Aug 22. Dermatol Ther (Heidelb). 2022. PMID: 35994159 Free PMC article.
-
New Developments in Topical Acne Therapy.Am J Clin Dermatol. 2022 Mar;23(2):125-136. doi: 10.1007/s40257-021-00666-9. Epub 2022 Jan 18. Am J Clin Dermatol. 2022. PMID: 35041198 Review.
-
Effect of Sarecycline on the Acne Symptom and Impact Scale and Concerns in Moderate-to-Severe Truncal Acne in Open-Label Pilot Study.Antibiotics (Basel). 2023 Jan 5;12(1):94. doi: 10.3390/antibiotics12010094. Antibiotics (Basel). 2023. PMID: 36671294 Free PMC article.
-
Trifarotene: First Approval.Drugs. 2019 Nov;79(17):1905-1909. doi: 10.1007/s40265-019-01218-6. Drugs. 2019. PMID: 31713811 Review.
-
A Real-World Approach to Trifarotene Treatment in Patients with Acne and Acne Sequelae Based on the Experience of the Italian Acne Board.Dermatol Ther (Heidelb). 2025 Feb;15(2):245-264. doi: 10.1007/s13555-024-01329-3. Epub 2025 Jan 9. Dermatol Ther (Heidelb). 2025. PMID: 39786713 Free PMC article.
References
-
- Tan JK, Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol 2015; 172(Suppl 1): 3–12. - PubMed
-
- Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol 1999; 41: 577–580. - PubMed
-
- Poli F, Dreno B, Verschoore M. An epidemiological study of acne in female adults: results of a survey conducted in France. J Eur Acad Dermatol Venereol 2001; 15: 541–545. - PubMed
-
- Perkins AC, Maglione J, Hillebrand GG, Miyamoto K, Kimball AB. Acne vulgaris in women: prevalence across the life span. J Women's Health 2002 2012; 21: 223–230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

