8-Chloroadenosine induces apoptosis in human coronary artery endothelial cells through the activation of the unfolded protein response
- PMID: 31307008
- PMCID: PMC6629973
- DOI: 10.1016/j.redox.2019.101274
8-Chloroadenosine induces apoptosis in human coronary artery endothelial cells through the activation of the unfolded protein response
Abstract
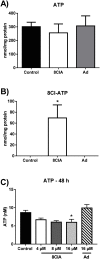
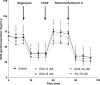
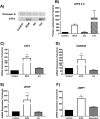
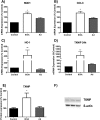
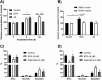
Infiltration of leukocytes within the vessel at sites of inflammation and the subsequent generation of myeloperoxidase-derived oxidants, including hypochlorous acid, are key characteristics of atherosclerosis. Hypochlorous acid is a potent oxidant that reacts readily with most biological molecules, including DNA and RNA. This results in nucleic acid modification and the formation of different chlorinated products. These products have been used as biomarkers of inflammation, owing to their presence in elevated amounts in different inflammatory fluids and diseased tissue, including atherosclerotic lesions. However, it is not clear whether these materials are simply biomarkers, or could also play a role in the development of chronic inflammatory pathologies. In this study, we examined the reactivity of different chlorinated nucleosides with human coronary artery endothelial cells (HCAEC). Evidence was obtained for the incorporation of each chlorinated nucleoside into the cellular RNA or DNA. However, only 8-chloro-adenosine (8ClA) had a significant effect on the cell viability and metabolic activity. Exposure of HCAEC to 8ClA decreased glycolysis, and resulted in a reduction in ATP, with a corresponding increase in the chlorinated analogue, 8Cl-ATP in the nucleotide pool. 8ClA also induced sustained endoplasmic reticulum stress within the HCAEC, which resulted in activation of the unfolded protein response, the altered expression of antioxidant genes and culminated in the release of calcium into the cytosol and cell death by apoptosis. Taken together, these data provide new insight into pathways by which myeloperoxidase activity and resultant hypochlorous acid generation could promote endothelial cell damage during chronic inflammation, which could be relevant to the progression of atherosclerosis.
Keywords: DNA; Hypochlorous acid; Inflammation; Myeloperoxidase; Nucleoside; RNA.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



Similar articles
-
8-Chloroadenosine Alters the Metabolic Profile and Downregulates Antioxidant and DNA Damage Repair Pathways in Macrophages.Chem Res Toxicol. 2020 Feb 17;33(2):402-413. doi: 10.1021/acs.chemrestox.9b00334. Epub 2019 Dec 16. Chem Res Toxicol. 2020. PMID: 31778309
-
Comparative reactivity of the myeloperoxidase-derived oxidants hypochlorous acid and hypothiocyanous acid with human coronary artery endothelial cells.Free Radic Biol Med. 2013 Dec;65:1352-1362. doi: 10.1016/j.freeradbiomed.2013.10.007. Epub 2013 Oct 10. Free Radic Biol Med. 2013. PMID: 24120969
-
A Role for Chlorinated Nucleosides in the Perturbation of Macrophage Function and Promotion of Inflammation.Chem Res Toxicol. 2019 Jun 17;32(6):1223-1234. doi: 10.1021/acs.chemrestox.9b00044. Epub 2019 May 16. Chem Res Toxicol. 2019. PMID: 31066272
-
Endothelial mitochondria and heart disease.Cardiovasc Res. 2010 Oct 1;88(1):58-66. doi: 10.1093/cvr/cvq195. Epub 2010 Jun 16. Cardiovasc Res. 2010. PMID: 20558442 Review.
-
Oxidant-specific biomarkers of oxidative stress. Association with atherosclerosis and implication for antioxidant effects.Free Radic Biol Med. 2018 May 20;120:425-440. doi: 10.1016/j.freeradbiomed.2018.04.001. Epub 2018 Apr 4. Free Radic Biol Med. 2018. PMID: 29625172 Review.
Cited by
-
Hypoxia Affects Autophagy in Human Umbilical Vein Endothelial Cells via the IRE1 Unfolded Protein Response.Curr Med Sci. 2023 Aug;43(4):689-695. doi: 10.1007/s11596-023-2749-y. Epub 2023 Aug 10. Curr Med Sci. 2023. PMID: 37558862
-
ADAR-Mediated A>I(G) RNA Editing in the Genotoxic Drug Response of Breast Cancer.Int J Mol Sci. 2024 Jul 6;25(13):7424. doi: 10.3390/ijms25137424. Int J Mol Sci. 2024. PMID: 39000531 Free PMC article. Review.
-
A Photoelectrochemical Sensor for the Detection of Hypochlorous Acid with a Phenothiazine-Based Photosensitizer.Molecules. 2024 Jan 27;29(3):614. doi: 10.3390/molecules29030614. Molecules. 2024. PMID: 38338358 Free PMC article.
-
SERCA Overexpression Improves Mitochondrial Quality Control and Attenuates Cardiac Microvascular Ischemia-Reperfusion Injury.Mol Ther Nucleic Acids. 2020 Sep 16;22:696-707. doi: 10.1016/j.omtn.2020.09.013. eCollection 2020 Dec 4. Mol Ther Nucleic Acids. 2020. Retraction in: Mol Ther Nucleic Acids. 2025 Jul 21;36(3):102637. doi: 10.1016/j.omtn.2025.102637. PMID: 33230467 Free PMC article. Retracted.
-
Natural products and other inhibitors of F1FO ATP synthase.Eur J Med Chem. 2020 Dec 1;207:112779. doi: 10.1016/j.ejmech.2020.112779. Epub 2020 Sep 3. Eur J Med Chem. 2020. PMID: 32942072 Free PMC article. Review.
References
-
- Davies M.J., Hawkins C.L., Pattison D.I., Rees M.D. Mammalian heme peroxidases: from molecular mechanisms to health implications. Antioxidants Redox Signal. 2008;10(7):1199–1234. - PubMed
-
- Rayner B.S., Love D.T., Hawkins C.L. Comparative reactivity of myeloperoxidase-derived oxidants with mammalian cells. Free Radic. Biol. Med. 2014;71:240–255. - PubMed
-
- Zhang R., Brennan M.L., Fu X., Aviles R.J., Pearce G.L., Penn M.S., Topol E.J., Sprecher D.L., Hazen S.L. Association between myeloperoxidase levels and risk of coronary artery disease. J. Am. Med. Assoc. 2001;286(17):2136–2142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

