Dynamic Compressive Loading Improves Cartilage Repair in an In Vitro Model of Microfracture: Comparison of 2 Mechanical Loading Regimens on Simulated Microfracture Based on Fibrin Gel Scaffolds Encapsulating Connective Tissue Progenitor Cells
- PMID: 31307219
- PMCID: PMC6637720
- DOI: 10.1177/0363546519855645
Dynamic Compressive Loading Improves Cartilage Repair in an In Vitro Model of Microfracture: Comparison of 2 Mechanical Loading Regimens on Simulated Microfracture Based on Fibrin Gel Scaffolds Encapsulating Connective Tissue Progenitor Cells
Abstract
Background: Microfracture of focal chondral defects often produces fibrocartilage, which inconsistently integrates with the surrounding native tissue and possesses inferior mechanical properties compared with hyaline cartilage. Mechanical loading modulates cartilage during development, but it remains unclear how loads produced in the course of postoperative rehabilitation affect the formation of the new fibrocartilaginous tissue.
Purpose: To assess the influence of different mechanical loading regimens, including dynamic compressive stress or rotational shear stress, on an in vitro model of microfracture repair based on fibrin gel scaffolds encapsulating connective tissue progenitor cells.
Study design: Controlled laboratory study.
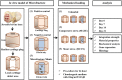
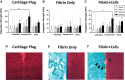
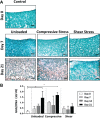
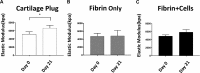
Methods: Cylindrical cores were made in bovine hyaline cartilage explants and filled with either (1) cartilage plug returned to original location (positive control), (2) fibrin gel (negative control), or (3) fibrin gel with encapsulated connective tissue progenitor cells (microfracture mimic). Constructs were then subjected to 1 of 3 loading regimens: (1) no loading (ie, unloaded), (2) dynamic compressive loading, or (3) rotational shear loading. On days 0, 7, 14, and 21, the integration strength between the outer chondral ring and the central insert was measured with an electroforce mechanical tester. The central core component, mimicking microfracture neotissue, was also analyzed for gene expression by real-time reverse-transcription polymerase chain reaction, glycosaminoglycan, and double-stranded DNA contents, and tissue morphology was analyzed histologically.
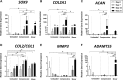
Results: Integration strengths between the outer chondral ring and central neotissue of the cartilage plug and fibrin + cells groups significantly increased upon exposure to compressive loading compared with day 0 controls (P = .007). Compressive loading upregulated expression of chondrogenesis-associated genes (SRY-related HGMG box-containing gene 9 [SOX9], collagen type II α1 [COL2A1], and increased ratio of COL2A1 to collagen type I α1 [COL1A1], an indicator of more hyaline phenotype) in the neotissue of the fibrin + cells group compared with the unloaded group at day 21 (SOX9, P = .0032; COL2A1, P < .0001; COL2A1:COL1A1, P = .0308). Fibrin + cells constructs exposed to shear loading expressed higher levels of chondrogenic genes compared with the unloaded condition, but the levels were not as high as those for the compressive loading condition. Furthermore, catabolic markers (MMP3 and ADAMTS 5) were significantly upregulated by shear loading (P = .0234 and P < .0001, respectively) at day 21 compared with day 0.
Conclusion: Dynamic compressive loading enhanced neotissue chondrogenesis and maturation in a simulated in vitro model of microfracture, with generation of more hyaline-like cartilage and improved integration with the surrounding tissue.
Clinical relevance: Controlled loading after microfracture may be beneficial in promoting the formation of more hyaline-like cartilage repair tissue; however, the loading regimens applied in this in vitro model do not yet fully reproduce the complex loading patterns created during clinical rehabilitation. Further optimization of in vitro models of cartilage repair may ultimately inform rehabilitation protocols.
Keywords: articular cartilage; connective tissue progenitor cells; mechanical loading; microfracture; rehabilitation.
Conflict of interest statement
One or more of the authors has declared the following potential conflict of interest or source of funding: R.S.T. received the MATE system free of charge as a beta tester from Apex Biomedical LLC (materials from a company that might benefit from this study). This research received funding from the Alliance for Regenerative Rehabilitation Research and Training (AR3T), which is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute of Neurological Disorders and Stroke (NINDS), and National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health (NIH) under award number P2CHD086843. This work is also supported, in part, by the US Department of Defense (W81XWH-14-2-0003 to R.S.T), by the EU Horizon 2020 - Research and Innovation Action SC1-PM 17 - 2017 +Project OACTIVE (under Grant Agreement No. 777159 to R.G.), and by Fondazione Ri.MED (grant to R.G.). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Figures


Similar articles
-
Enhanced chondrogenic phenotype of primary bovine articular chondrocytes in Fibrin-Hyaluronan hydrogel by multi-axial mechanical loading and FGF18.Acta Biomater. 2020 Mar 15;105:170-179. doi: 10.1016/j.actbio.2020.01.032. Epub 2020 Jan 23. Acta Biomater. 2020. PMID: 31982592
-
Mechanically stimulated osteochondral organ culture for evaluation of biomaterials in cartilage repair studies.Acta Biomater. 2018 Nov;81:256-266. doi: 10.1016/j.actbio.2018.09.058. Epub 2018 Sep 28. Acta Biomater. 2018. PMID: 30273741
-
Redifferentiated Chondrocytes in Fibrin Gel for the Repair of Articular Cartilage Lesions.Am J Sports Med. 2019 Aug;47(10):2348-2359. doi: 10.1177/0363546519857571. Epub 2019 Jul 2. Am J Sports Med. 2019. PMID: 31265317
-
Regulation of the chondrogenic phenotype in culture.Birth Defects Res C Embryo Today. 2009 Dec;87(4):351-71. doi: 10.1002/bdrc.20167. Birth Defects Res C Embryo Today. 2009. PMID: 19960542 Review.
-
Mechanobiological conditioning of stem cells for cartilage tissue engineering.Biomed Mater Eng. 2006;16(4 Suppl):S37-52. Biomed Mater Eng. 2006. PMID: 16823112 Review.
Cited by
-
Aggrecan and Hyaluronan: The Infamous Cartilage Polyelectrolytes - Then and Now.Adv Exp Med Biol. 2023;1402:3-29. doi: 10.1007/978-3-031-25588-5_1. Adv Exp Med Biol. 2023. PMID: 37052843
-
Effects of and Response to Mechanical Loading on the Knee.Sports Med. 2022 Feb;52(2):201-235. doi: 10.1007/s40279-021-01579-7. Epub 2021 Oct 20. Sports Med. 2022. PMID: 34669175 Review.
-
Compression Bioreactor-Based Mechanical Loading Induces Mobilization of Human Bone Marrow-Derived Mesenchymal Stromal Cells into Collagen Scaffolds In Vitro.Int J Mol Sci. 2020 Nov 4;21(21):8249. doi: 10.3390/ijms21218249. Int J Mol Sci. 2020. PMID: 33158020 Free PMC article.
-
The advances in nanomedicine for bone and cartilage repair.J Nanobiotechnology. 2022 Mar 18;20(1):141. doi: 10.1186/s12951-022-01342-8. J Nanobiotechnology. 2022. PMID: 35303876 Free PMC article. Review.
-
Recent Developments and Current Applications of Organic Nanomaterials in Cartilage Repair.Bioengineering (Basel). 2022 Aug 15;9(8):390. doi: 10.3390/bioengineering9080390. Bioengineering (Basel). 2022. PMID: 36004915 Free PMC article. Review.
References
-
- Bensaid W, Triffitt JT, Blanchat C, Oudina K, Sedel L, Petite H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24(14):2497-2502. - PubMed
-
- Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res. 2004;427suppl):S6-S15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

