Assessment of the abstract reporting of systematic reviews of dose-response meta-analysis: a literature survey
- PMID: 31307388
- PMCID: PMC6631883
- DOI: 10.1186/s12874-019-0798-5
Assessment of the abstract reporting of systematic reviews of dose-response meta-analysis: a literature survey
Abstract
Background: There is an increasing number of published systematic reviews (SR) of dose-response meta-analyses (DRMAs) over the past decades. However, the quality of abstract reporting of these SR-DRMAs remains to be understood. We conducted a literature survey to investigate the abstract reporting of SR-DRMAs.
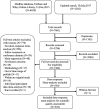
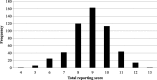
Methods: Medline, Embase, and Wiley online Library were searched for eligible SR-DRMAs. The reporting quality of SR-DRMAs was assessed by the modified PRISMA-for-Abstract checklist (14 items). We summarized the adherence rate of each item and categorized them as well complied (adhered by 80% or above), moderately complied (50 to 79%), and poorly complied (less than 50%). We used total score to reflect the abstract quality and regression analysis was employed to explore the potential influence factors for it.
Results: We included 529 SR-DRMAs. Eight of 14 items were moderately (3 items) or poorly complied (5 items) while only 6 were well complied by these SR-DRMAs. Most of the SR-DRMAs failed to describe the methods for risk of bias assessment (30.2, 95% CI: 26.4, 34.4%) and the results of bias assessment (48.8, 95% CI: 44.4, 53.1%). Few SR-DRMAs reported the funding (2.3, 95% CI: 1.2, 3.9%) and registration (0.6, 95% CI: 0.1, 1.6%) information in the abstract. Multivariable regression analysis suggested word number of abstracts [> 250 vs. ≤ 250 (estimated ß = 0.31; 95% CI: 0.02, 0.61; P = 0.039)] was positively associated with the abstract reporting quality.
Conclusion: The abstract reporting of SR-DRMAs is suboptimal, substantial effort is needed to improve the reporting. More word number may benefit for the abstract reporting. Given that reporting of abstract largely depends on the reporting and conduct of the SR-DRMA, review authors should also focus on the completeness of SR-DRMA itself.
Keywords: Abstract reporting; Dose-response meta-analysis; Literature survey; Systematic review.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Improving the quality of reporting of systematic reviews of dose-response meta-analyses: a cross-sectional survey.BMC Med Res Methodol. 2018 Nov 29;18(1):157. doi: 10.1186/s12874-018-0623-6. BMC Med Res Methodol. 2018. PMID: 30497389 Free PMC article.
-
Protocol registration or development may benefit the design, conduct and reporting of dose-response meta-analysis: empirical evidence from a literature survey.BMC Med Res Methodol. 2019 Apr 11;19(1):78. doi: 10.1186/s12874-019-0715-y. BMC Med Res Methodol. 2019. PMID: 30975073 Free PMC article.
-
Abstract analysis method facilitates filtering low-methodological quality and high-bias risk systematic reviews on psoriasis interventions.BMC Med Res Methodol. 2017 Dec 29;17(1):180. doi: 10.1186/s12874-017-0460-z. BMC Med Res Methodol. 2017. PMID: 29284417 Free PMC article.
-
Systematic reviews in orthodontics: Impact of the PRISMA for Abstracts checklist on completeness of reporting.Am J Orthod Dentofacial Orthop. 2019 Oct;156(4):442-452.e12. doi: 10.1016/j.ajodo.2019.05.009. Am J Orthod Dentofacial Orthop. 2019. PMID: 31582116 Review.
-
A scoping review of comparisons between abstracts and full reports in primary biomedical research.BMC Med Res Methodol. 2017 Dec 29;17(1):181. doi: 10.1186/s12874-017-0459-5. BMC Med Res Methodol. 2017. PMID: 29287585 Free PMC article.
Cited by
-
Oral Health Care Among Women in Perimenopause or Menopause: An Integrative Review.J Midwifery Womens Health. 2025 Jan-Feb;70(1):17-31. doi: 10.1111/jmwh.13668. Epub 2024 Jul 24. J Midwifery Womens Health. 2025. PMID: 39045880 Free PMC article. Review.
-
Vaccination for Human Papillomavirus: an historic and bibliometric study.Hum Vaccin Immunother. 2021 Apr 3;17(4):934-942. doi: 10.1080/21645515.2020.1805991. Epub 2020 Sep 21. Hum Vaccin Immunother. 2021. PMID: 32955407 Free PMC article.
References
-
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Epidemiol Biostatistics Public Health. 2009;6(4):e1–e34. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

