Upregulation of cell-surface mucin MUC15 in human nasal epithelial cells upon influenza A virus infection
- PMID: 31307416
- PMCID: PMC6631914
- DOI: 10.1186/s12879-019-4213-y
Upregulation of cell-surface mucin MUC15 in human nasal epithelial cells upon influenza A virus infection
Abstract
Background: Cell-surface mucins are expressed in apical epithelial cells of the respiratory tract, and contribute a crucial part of the innate immune system. Despite anti-inflammatory or antiviral functions being revealed for certain cell-surface mucins such as MUC1, the roles of other mucins are still poorly understood, especially in viral infections.
Methods: To further identify mucins significant in influenza infection, we screened the expression of mucins in human nasal epithelial cells infected by H3N2 influenza A virus.
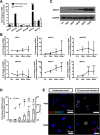
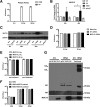
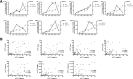
Results: We found that the expression of MUC15 was significantly upregulated upon infection, and specific only to active infection. While MUC15 did not interact with virus particles or reduce viral replication directly, positive correlations were observed between MUC15 and inflammatory factors in response to viral infection. Given that the upregulation of MUC15 was only triggered late into infection when immune factors (including cytokines, chemokines, EGFR and phosphorylated ERK) started to peak and plateau, MUC15 may potentially serve an immunomodulatory function later during influenza viral infection.
Conclusions: Our study revealed that MUC15 was one of the few cell-surface mucins induced during influenza infection. While MUC15 did not interact directly with influenza virus, we showed that its increase coincides with the peak of immune activation and thus MUC15 may serve an immunomodulatory role during influenza infection.
Keywords: H3N2; Immunomodulation; MUC15; Mucin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Infection and Replication of Influenza Virus at the Ocular Surface.J Virol. 2018 Mar 14;92(7):e02192-17. doi: 10.1128/JVI.02192-17. Print 2018 Apr 1. J Virol. 2018. PMID: 29321303 Free PMC article.
-
Membrane-Tethered Mucin 1 Is Stimulated by Interferon and Virus Infection in Multiple Cell Types and Inhibits Influenza A Virus Infection in Human Airway Epithelium.mBio. 2022 Aug 30;13(4):e0105522. doi: 10.1128/mbio.01055-22. Epub 2022 Jun 14. mBio. 2022. PMID: 35699372 Free PMC article.
-
RNA Sequencing of H3N2 Influenza Virus-Infected Human Nasal Epithelial Cells from Multiple Subjects Reveals Molecular Pathways Associated with Tissue Injury and Complications.Cells. 2019 Aug 27;8(9):986. doi: 10.3390/cells8090986. Cells. 2019. PMID: 31461941 Free PMC article.
-
Targeting MUC15 Protein in Cancer: Molecular Mechanisms and Therapeutic Perspectives.Curr Cancer Drug Targets. 2020;20(9):647-653. doi: 10.2174/1568009620666200601140639. Curr Cancer Drug Targets. 2020. PMID: 32479243 Review.
-
Mucin-interacting proteins: from function to therapeutics.Trends Biochem Sci. 2010 Apr;35(4):236-45. doi: 10.1016/j.tibs.2009.10.003. Epub 2009 Nov 11. Trends Biochem Sci. 2010. PMID: 19913432 Free PMC article. Review.
Cited by
-
Exercise duration modulates upper and lower respiratory fluid cellularity, antiviral activity, and lung gene expression.Physiol Rep. 2021 Oct;9(20):e15075. doi: 10.14814/phy2.15075. Physiol Rep. 2021. PMID: 34676696 Free PMC article.
-
An unexpected biomaterial against SARS-CoV-2: Bio-polyphosphate blocks binding of the viral spike to the cell receptor.Mater Today (Kidlington). 2021 Dec;51:504-524. doi: 10.1016/j.mattod.2021.07.029. Epub 2021 Aug 2. Mater Today (Kidlington). 2021. PMID: 34366696 Free PMC article. Review.
-
Novel ACE2 protein interactions relevant to COVID-19 predicted by evolutionary rate correlations.PeerJ. 2021 Sep 15;9:e12159. doi: 10.7717/peerj.12159. eCollection 2021. PeerJ. 2021. PMID: 34616619 Free PMC article.
-
Insights into Lichen Planus Pigmentosus Inversus using Minimally Invasive Dermal Patch and Whole Transcriptome Analysis.J Clin Investig Dermatol. 2022;10(1):10.13188/2373-1044.1000077. doi: 10.13188/2373-1044.1000077. Epub 2022 Apr 5. J Clin Investig Dermatol. 2022. PMID: 36003415 Free PMC article.
-
Meta-analysis of global and high throughput public gene array data for robust vascular gene expression discovery in chronic rhinosinusitis: Implications in controlled release.J Control Release. 2021 Feb 10;330:878-888. doi: 10.1016/j.jconrel.2020.10.061. Epub 2020 Nov 2. J Control Release. 2021. PMID: 33144181 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

