Animal to human translation: a systematic scoping review of reported concordance rates
- PMID: 31307492
- PMCID: PMC6631915
- DOI: 10.1186/s12967-019-1976-2
Animal to human translation: a systematic scoping review of reported concordance rates
Abstract
Background: Drug development is currently hampered by high attrition rates; many developed treatments fail during clinical testing. Part of the attrition may be due to low animal-to-human translational success rates; so-called "translational failure". As far as we know, no systematic overview of published translational success rates exists.
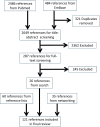
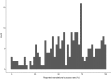
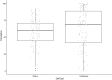
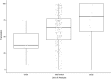
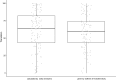
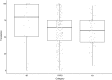
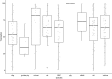
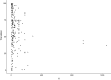
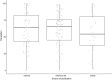
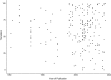
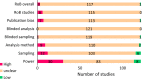
Systematic scoping review: The following research question was examined: "What is the observed range of the animal-to-human translational success (and failure) rates within the currently available empirical evidence?". We searched PubMed and Embase on 16 October 2017. We included reviews and all other types of "umbrella"-studies of meta-data quantitatively comparing the translational results of studies including at least two species with one being human. We supplemented our database searches with additional strategies. All abstracts and full-text papers were screened by two independent reviewers. Our scoping review comprises 121 references, with various units of measurement: compound or intervention (k = 104), study/experiment (k = 10), and symptom or event (k = 7). Diagnostic statistics corresponded with binary and continuous definitions of successful translation. Binary definitions comprise percentages below twofold error, percentages accurately predicted, and predictive values. Quantitative definitions comprise correlation/regression (r2) and meta-analyses (percentage overlap of 95% confidence intervals). Translational success rates ranged from 0 to 100%.
Conclusion: The wide range of translational success rates observed in our study might indicate that translational success is unpredictable; i.e. it might be unclear upfront if the results of primary animal studies will contribute to translational knowledge. However, the risk of bias of the included studies was high, and much of the included evidence is old, while newer models have become available. Therefore, the reliability of the cumulative evidence from current papers on this topic is insufficient. Further in-depth "umbrella"-studies of translational success rates are still warranted. These are needed to evaluate the probabilistic evidence for predictivity of animal studies for the human situation more reliably, and to determine which factors affect this process.
Keywords: Prediction; Systematic review; Translation.
Conflict of interest statement
Merel Ritskes-Hoitinga MR-H is a member of the council of management of the UK registered company/charity Laboratory Animals Ltd (LAL). LAL issues the journal Laboratory Animals. The position is unpaid but travel to LAL meetings is reimbursed. The journal’s profits are used for charitable purposes, subsidising educational projects in laboratory animal science and welfare. The other authors have no competing interests.
Figures
References
-
- Steedman M, Taylor K, Stockbridge M, Korba C, DShah S, Thaxter M. Unlocking R&D productivity—measuring the return from pharmaceutical innovation 2018. 2019.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

