A dose-ranging, parallel group, split-face, single-blind phase II study of light emitting diode-red light (LED-RL) for skin scarring prevention: study protocol for a randomized controlled trial
- PMID: 31307501
- PMCID: PMC6631489
- DOI: 10.1186/s13063-019-3546-6
A dose-ranging, parallel group, split-face, single-blind phase II study of light emitting diode-red light (LED-RL) for skin scarring prevention: study protocol for a randomized controlled trial
Abstract
Background: Skin fibrosis is a significant global health problem that affects over 100 million people annually and has a profoundly negative impact on quality of life. Characterized by excessive fibroblast proliferation and collagen deposition, skin fibrosis underlies a wide spectrum of dermatologic conditions ranging from pathologic scars secondary to injury (e.g., burns, surgery, trauma) to immune-mediated diseases. Effective anti-scarring therapeutics remain an unmet need, underscoring the importance of developing novel approaches to treat and prevent skin fibrosis. Our in vitro data show that light emitting diode-red light (LED-RL) can modulate key cellular and molecular processes involved in skin fibrosis. In two phase I clinical trials (STARS 1 and STARS 2), we demonstrated the safety and tolerability of LED-RL at fluences of 160 J/cm2 up to 480 J/cm2 on normal human skin.
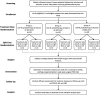
Methods/design: CURES (Cutaneous Understanding of Red-light Efficacy on Scarring) is a dose-ranging, randomized, parallel group, split-face, single-blind, mock-controlled phase II study to evaluate the efficacy of LED-RL to limit post-surgical skin fibrosis in subjects undergoing elective mini-facelift surgery. Thirty subjects will be randomly allocated to three treatment groups to receive LED-RL phototherapy or temperature-matched mock irradiation (control) to either periauricular incision site at fluences of 160 J/cm2, 320 J/cm2, or 480 J/cm2. Starting one week post-surgery (postoperative days 4-8), treatments will be administered three times weekly for three consecutive weeks, followed by efficacy assessments at 30 days, 3 months, and 6 months. The primary endpoint is the difference in scar pliability between LED-RL-treated and control sites as determined by skin elasticity and induration measurements. Secondary outcomes include clinical and photographic evaluations of scars, 3D skin imaging analysis, histological and molecular analyses, and adverse events.
Discussion: LED-RL is a therapeutic modality of increasing importance in dermatology, and has the potential to limit skin fibrosis clinically by decreasing dermal fibroblast activity and collagen production. The administration of LED-RL phototherapy in the early postoperative period may optimize wound healing and prevent excessive scarring. The results from this study may change the current treatment paradigm for fibrotic skin diseases and help to pioneer LED-RL as a safe, non-invasive, cost-effective, portable, at-home therapy for scars.
Trial registration: ClinicalTrials.gov, NCT03795116 . Registered on 20 December 2018.
Keywords: Hypertrophic scar; Keloid; Light emitting diode; Phototherapy; Red light; Scarring; Skin fibrosis; Surgery; Wound healing.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Light emitting diode-red light for reduction of post-surgical scarring: Results from a dose-ranging, split-face, randomized controlled trial.J Biophotonics. 2021 Jul;14(7):e202100073. doi: 10.1002/jbio.202100073. Epub 2021 May 4. J Biophotonics. 2021. PMID: 33788987 Free PMC article. Clinical Trial.
-
A single-blind, dose escalation, phase I study of high-fluence light-emitting diode-red light (LED-RL) on human skin: study protocol for a randomized controlled trial.Trials. 2016 Aug 2;17:385. doi: 10.1186/s13063-016-1518-7. Trials. 2016. PMID: 27484782 Free PMC article. Clinical Trial.
-
A single-blind, dose-escalation, phase I study of high-fluence light-emitting diode-red light on Caucasian non-Hispanic skin: study protocol for a randomized controlled trial.Trials. 2019 Mar 20;20(1):177. doi: 10.1186/s13063-019-3278-7. Trials. 2019. PMID: 30894210 Free PMC article.
-
Visible Red Light Emitting Diode Photobiomodulation for Skin Fibrosis: Key Molecular Pathways.Curr Dermatol Rep. 2016;5:121-128. doi: 10.1007/s13671-016-0141-x. Epub 2016 Apr 16. Curr Dermatol Rep. 2016. PMID: 27182462 Free PMC article. Review.
-
Scar-free healing: from embryonic mechanisms to adult therapeutic intervention.Philos Trans R Soc Lond B Biol Sci. 2004 May 29;359(1445):839-50. doi: 10.1098/rstb.2004.1475. Philos Trans R Soc Lond B Biol Sci. 2004. PMID: 15293811 Free PMC article. Review.
Cited by
-
Use of a novel indentometer to evaluate skin stiffness in healthy and diseased human skin.Skin Res Technol. 2023 Jul;29(7):e13384. doi: 10.1111/srt.13384. Skin Res Technol. 2023. PMID: 37522487 Free PMC article.
-
Light emitting diode-red light for reduction of post-surgical scarring: Results from a dose-ranging, split-face, randomized controlled trial.J Biophotonics. 2021 Jul;14(7):e202100073. doi: 10.1002/jbio.202100073. Epub 2021 May 4. J Biophotonics. 2021. PMID: 33788987 Free PMC article. Clinical Trial.
-
Using Durometers to Quantify Stiffness as an Outcome Measure for Cutaneous Neurofibroma in Neurofibromatosis Type 1.JID Innov. 2025 May 12;5(4):100380. doi: 10.1016/j.xjidi.2025.100380. eCollection 2025 Jul. JID Innov. 2025. PMID: 40529476 Free PMC article.
-
High-fluence light emitting diode-red light inhibits cell cycle progression in human dermal fibroblasts.J Biophotonics. 2021 Feb;14(2):e202000359. doi: 10.1002/jbio.202000359. Epub 2020 Nov 3. J Biophotonics. 2021. PMID: 33038043 Free PMC article.
-
Cellular and Molecular Processes in Wound Healing.Biomedicines. 2023 Sep 13;11(9):2526. doi: 10.3390/biomedicines11092526. Biomedicines. 2023. PMID: 37760967 Free PMC article. Review.
References
-
- Luzina IG, Atamas SP. Fibrotic skin diseases. In: Gaspari A, Tyring S, editors. Clinical and basic immunodermatology. London: Springer London; 2008. pp. 721–737.