Safety, effectiveness, and economic evaluation of an herbal medicine, Ukgansangajinpibanha granule, in children with autism spectrum disorder: a study protocol for a prospective, multicenter, randomized, double-blinded, placebo-controlled, parallel-group clinical trial
- PMID: 31307524
- PMCID: PMC6631544
- DOI: 10.1186/s13063-019-3537-7
Safety, effectiveness, and economic evaluation of an herbal medicine, Ukgansangajinpibanha granule, in children with autism spectrum disorder: a study protocol for a prospective, multicenter, randomized, double-blinded, placebo-controlled, parallel-group clinical trial
Abstract
Background: Autism spectrum disorder (ASD) is characterized by continuous impairment in communication and social interaction and by limited and repetitive behaviors, interests, or activities. Behavioral, educational, and pharmaceutical interventions have been shown to reduce behavioral disabilities, improve verbal/non-verbal communication, and help patients acquire self-reliance skills. However, there has been a lack of systematic verification and consensus regarding the treatment of the core symptoms of ASD because of its unclear etiology. Ukgansangajinpibanha (UGSJB), a legitimately prescribed herbal medicine for nervousness, insomnia, night crying, and malnutrition in South Korea and Japan, has been used for angry, sensitive, nervous, and unsettled children with ASD.
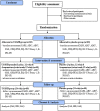
Methods/design: This trial is a prospective, multicenter, randomized, double-blinded, placebo-controlled, parallel-group, clinical trial. The 4- to 6-year-old children with ASD will be randomly assigned to following groups: 1. A UGSJB granule with acupuncture, twice daily (n = 120) 2. A placebo group with acupuncture, twice daily (n = 120). The following outcome measures will be used: behavior by the Childhood Autism Rating Scale, Autism Behavior Checklist, and Aberrant Behavior Checklist; social maturity by the Social Maturity Scale; quality of life by the Child Health Questionnaire and EuroQoL Five-dimension Five-level Youth; and parental stress by the Parenting Stress Index at baseline and at 6, 12, and 24 weeks after the beginning of treatment. In addition, to evaluate safety, we will investigate the adverse reactions that may be caused by UGSJB granule. Finally, we will make an economic evaluation of UGSJB for the treatment of ASD.
Discussion: We prepared a well-designed clinical trial to investigate the safety and effectiveness of UGSJB on ASD symptoms compared with placebo treatment. The results from this study will provide clinical evidence on the safety, effectiveness, and economic value of UGSJB combined with acupuncture in children with ASD.
Trial registration: Clinical Research Information Service: KCT0003007 (registered on April 5, 2018).
Keywords: Acupuncture; Autism spectrum disorder; Herbal medicine; Randomized controlled trial; Ukgansangajinpibanha.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Randomized controlled trial of vitamin D supplementation in children with autism spectrum disorder.J Child Psychol Psychiatry. 2018 Jan;59(1):20-29. doi: 10.1111/jcpp.12652. Epub 2016 Nov 21. J Child Psychol Psychiatry. 2018. Retraction in: J Child Psychol Psychiatry. 2019 Jun;60(6):711. doi: 10.1111/jcpp.13076. PMID: 27868194 Retracted. Clinical Trial.
-
Rationale and design for cognitive behavioral therapy for anxiety disorders in children with autism spectrum disorder: a study protocol of a randomized controlled trial.Trials. 2018 Apr 2;19(1):210. doi: 10.1186/s13063-018-2591-x. Trials. 2018. PMID: 29609630 Free PMC article.
-
Glutamatergic medication in the treatment of obsessive compulsive disorder (OCD) and autism spectrum disorder (ASD) - study protocol for a randomised controlled trial.Trials. 2016 Mar 17;17(1):141. doi: 10.1186/s13063-016-1266-8. Trials. 2016. PMID: 26983548 Free PMC article. Clinical Trial.
-
Bumetanide for Autism Spectrum Disorder in Children: A Review of Randomized Controlled Trials.Ann Pharmacother. 2019 May;53(5):537-544. doi: 10.1177/1060028018817304. Epub 2018 Dec 2. Ann Pharmacother. 2019. PMID: 30501497 Review.
-
Autism Spectrum Disorder: Classification, diagnosis and therapy.Pharmacol Ther. 2018 Oct;190:91-104. doi: 10.1016/j.pharmthera.2018.05.007. Epub 2018 May 12. Pharmacol Ther. 2018. PMID: 29763648 Review.
Cited by
-
Research status and prospects of acupuncture for autism spectrum disorders.Front Psychiatry. 2023 May 25;14:942069. doi: 10.3389/fpsyt.2023.942069. eCollection 2023. Front Psychiatry. 2023. PMID: 37304438 Free PMC article. Review.
-
Zebrafish Modeling of Autism Spectrum Disorders, Current Status and Future Prospective.Front Psychiatry. 2022 Jul 14;13:911770. doi: 10.3389/fpsyt.2022.911770. eCollection 2022. Front Psychiatry. 2022. PMID: 35911241 Free PMC article. Review.
-
A bibliometric analysis of acupuncture for neurodevelopmental disorders: A Call for increased output and future research priorities.Heliyon. 2023 Nov 23;9(12):e22799. doi: 10.1016/j.heliyon.2023.e22799. eCollection 2023 Dec. Heliyon. 2023. PMID: 38213582 Free PMC article. Review.
References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Seoul: Hakjisa; 2015.
-
- Christensen DL, Bilder DA, Zahorodny W, Pettygrove S, Durkin MS, Fitzgerald RT, et al. Prevalence and characteristics of autism spectrum disorder among 4-year-old children in the autism and developmental disabilities monitoring network. J Dev Behav Pediatrics. 2016;37:1–8. doi: 10.1097/DBP.0000000000000235. - DOI - PubMed
-
- Scottish Intercollegiate Guidelines Network (SIGN) Assessment, diagnosis and interventions for autism spectrum disorders. Edinburgh: SIGN; 2016.
-
- Textbook Compiliation Committee . Pediatrics of Korean Medicine. revised edn. Seoul: Euisungdang; 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

