The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC)
- PMID: 31307547
- PMCID: PMC6632213
- DOI: 10.1186/s40425-019-0662-5
The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC)
Abstract
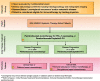
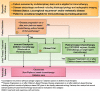
Head and neck cancers, including those of the lip and oral cavity, nasal cavity, paranasal sinuses, oropharynx, larynx and nasopharynx represent nearly 700,000 new cases and 380,000 deaths worldwide per annum, and account for over 10,000 annual deaths in the United States alone. Improvement in outcomes are needed for patients with recurrent and or metastatic squamous cell carcinoma of the head and neck (HNSCC). In 2016, the US Food and Drug Administration (FDA) granted the first immunotherapeutic approvals - the anti-PD-1 immune checkpoint inhibitors nivolumab and pembrolizumab - for the treatment of patients with recurrent squamous cell carcinoma of the head and neck (HNSCC) that is refractory to platinum-based regimens. The European Commission followed in 2017 with approval of nivolumab for treatment of the same patient population, and shortly thereafter with approval of pembrolizumab monotherapy for the treatment of recurrent or metastatic HNSCC in adults whose tumors express PD-L1 with a ≥ 50% tumor proportion score and have progressed on or after platinum-containing chemotherapy. Then in 2019, the FDA granted approval for PD-1 inhibition as first-line treatment for patients with metastatic or unresectable, recurrent HNSCC, approving pembrolizumab in combination with platinum and fluorouracil for all patients with HNSCC and pembrolizumab as a single agent for patients with HNSCC whose tumors express a PD-L1 combined positive score ≥ 1. These approvals marked the first new therapies for these patients since 2006, as well as the first immunotherapeutic approvals in this disease. In light of the introduction of these novel therapies for the treatment of patients with head and neck cancer, The Society for Immunotherapy of Cancer (SITC) formed an expert committee tasked with generating consensus recommendations for emerging immunotherapies, including appropriate patient selection, therapy sequence, response monitoring, adverse event management, and biomarker testing. These consensus guidelines serve as a foundation to assist clinicians' understanding of the role of immunotherapies in this disease setting, and to standardize utilization across the field for patient benefit. Due to country-specific variances in approvals, availability and regulations regarding the discussed agents, this panel focused solely on FDA-approved drugs for the treatment of patients in the U.S.
Keywords: Guidelines; Head and neck cancer; Head and neck squamous cell carcinoma (HNSCC); Immune checkpoint inhibitor (ICI); Immunotherapy.
Conflict of interest statement
RLF has participated on advisory boards for Amgen, AstraZeneca, MedImmune, Bristol-Myers Squibb, EMD Serono, PPD (Benitec, Immunicum), Eli Lilly, Merck, and Pfizer. In addition, he has received research funding/grant support from AstraZeneca, Medimmune, Bristol-Myers Squibb, PPD, Merck, and VentiRx Pharmaceuticals. EC has served as a consultant for Eisai, Pfizer, Merck, AstraZeneca, Bristol-Myers Squibb, and is a stock holder of Human Longevity, Inc. RBR has served as a consultant for Stryker and serves on the Speakers Bureau for Merck. He has also received research funding from Bristol-Myers Squibb and MedImmune. BAB has served on the advisory board and/or as a consultant for Amgen, Debiopharma, Celgene, Merck, Boehringer Ingelheim, Genentech, AstraZeneca, and Bristol-Myers Squibb. She has also received research funding from Advaxis, Merck, and Bristol-Myers Squibb. CBB holds stock ownership in PrimeVax and participated in scientific advisory boards for BMS, Roche, PrimeVax and HalioDx. MLG has served as a consultant for Bristol-Myers Squibb, Beyer Pharmaceuticals, Amgen, Genocea, Merck, EMD Serono, Celgene, GSK, AstraZeneca, Roche, Ventana and Aspyrian. MLG is on a DSMB for BioMimetics. KH has received honoraria, research funding, and/or speaker’s bureau fees paid to the institution from Amgen, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Merck, MSD, and Pfizer. RL has served as a consultant for AstraZeneca and Regeneron, and has received research funding from Bristol-Myers Squibb, MedImmune, and Ignyta. Additionally, his Wife, Dr. Lily Liu, is an employee of Agonox. LL has receiver honoraria and/or consulting fees from Eisai, Bristol-Myers Squibb, MSD, Merck-Serono, Boehringer Ingelheim, Novartis, Astrazeneca, Roche, Bayer, Debiopharm and Sobi, as well as research funding from Eisai, MSD, Merck-Serono, Boehringer Ingelheim, Novartis, AstraZeneca, and Roche. HM has received research funding from GlaxoSmithKline, MSD, Sanofi Pasteur, GSK PLC, AstraZeneca, and Silence Therapeutics, and is a stock holder of Warwickshire Head Neck Clinic. LKM has received honoraria from Varian, served as a consultant for Bristol-Myers Squibb, and has received research funding from Merck and AstraZeneca. AR has served as a consultant and is on the advisory board for Bristol-Myers Squibb, and has served as a consultant and has served on the speaker’s bureau for FMI. AS has received research funding from Advaxis, LLC. and Tessa Therapeutics. RU has served on the advisory board for Merck. DPZ has served as a principle investigator for clinical trials supported by Bristol-Myers Squibb, Merck, AztraZeneca, MedImmune, Gliknik, and Macrogenics. NYL is on the advisory board/consultant for Merck, Merck-Serono Bristol-Myers Squibb, Sanofi Aventis, Lily, Pfister, Vertex. QTL, RLL, and FW declare no competing interests.
Figures