α4β1 integrin associates with VEGFR2 in CLL cells and contributes to VEGF binding and intracellular signaling
- PMID: 31307975
- PMCID: PMC6650728
- DOI: 10.1182/bloodadvances.2019000019
α4β1 integrin associates with VEGFR2 in CLL cells and contributes to VEGF binding and intracellular signaling
Abstract
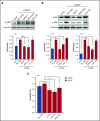
α4β1 integrin and VEGFR2 function as a receptor complex for VEGF in CLL cells.
Contribution to VEGF functions in CLL is a novel pathological role for α4β1 integrin in this malignancy.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

Similar articles
-
VEGF/VEGFR2 interaction down-regulates matrix metalloproteinase-9 via STAT1 activation and inhibits B chronic lymphocytic leukemia cell migration.Blood. 2010 Jan 28;115(4):846-9. doi: 10.1182/blood-2009-08-239426. Epub 2009 Nov 19. Blood. 2010. PMID: 19965686
-
CLL, but not normal, B cells are dependent on autocrine VEGF and alpha4beta1 integrin for chemokine-induced motility on and through endothelium.Blood. 2005 Jun 15;105(12):4813-9. doi: 10.1182/blood-2004-10-4054. Epub 2005 Feb 24. Blood. 2005. PMID: 15731179
-
A 17-residue sequence from the matrix metalloproteinase-9 (MMP-9) hemopexin domain binds α4β1 integrin and inhibits MMP-9-induced functions in chronic lymphocytic leukemia B cells.J Biol Chem. 2012 Aug 10;287(33):27601-13. doi: 10.1074/jbc.M112.354670. Epub 2012 Jun 22. J Biol Chem. 2012. PMID: 22730324 Free PMC article. Clinical Trial.
-
Functional and Clinical Significance of the Integrin Alpha Chain CD49d Expression in Chronic Lymphocytic Leukemia.Curr Cancer Drug Targets. 2016;16(8):659-668. doi: 10.2174/1568009616666160809102219. Curr Cancer Drug Targets. 2016. PMID: 27514846 Review.
-
Survival of chronic lymphocytic leukemia cells: CD40L and the vascular endothelial growth factor (VEGF) connection.Leukemia. 2005 Apr;19(4):531-2. doi: 10.1038/sj.leu.2403677. Leukemia. 2005. PMID: 15861177 Review. No abstract available.
Cited by
-
Growth Factors VEGF-A165 and FGF-2 as Multifunctional Biomolecules Governing Cell Adhesion and Proliferation.Int J Mol Sci. 2021 Feb 12;22(4):1843. doi: 10.3390/ijms22041843. Int J Mol Sci. 2021. PMID: 33673317 Free PMC article.
-
Comparative Approach to the Temporo-Spatial Organization of the Tumor Microenvironment.Front Oncol. 2019 Nov 7;9:1185. doi: 10.3389/fonc.2019.01185. eCollection 2019. Front Oncol. 2019. PMID: 31788448 Free PMC article. Review.
-
Tumor Cell Survival Factors and Angiogenesis in Chronic Lymphocytic Leukemia: How Hot Is the Link?Cancers (Basel). 2024 Dec 29;17(1):72. doi: 10.3390/cancers17010072. Cancers (Basel). 2024. PMID: 39796700 Free PMC article. Review.
-
Vascular endothelial growth factor signaling in health and disease: from molecular mechanisms to therapeutic perspectives.Signal Transduct Target Ther. 2025 May 19;10(1):170. doi: 10.1038/s41392-025-02249-0. Signal Transduct Target Ther. 2025. PMID: 40383803 Free PMC article. Review.
-
Relation of Neutrophil Gelatinase-Associated Lipocalin Overexpression to the Resistance to Apoptosis of Tumor B Cells in Chronic Lymphocytic Leukemia.Cancers (Basel). 2020 Jul 31;12(8):2124. doi: 10.3390/cancers12082124. Cancers (Basel). 2020. PMID: 32751884 Free PMC article.
References
-
- Lagneaux L, Delforge A, Bron D, De Bruyn C, Stryckmans P. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood. 1998;91(7):2387-2396. - PubMed
-
- de la Fuente MT, Casanova B, Moyano JV, et al. . Engagement of alpha4beta1 integrin by fibronectin induces in vitro resistance of B chronic lymphocytic leukemia cells to fludarabine. J Leukoc Biol. 2002;71(3):495-502. - PubMed
-
- Lee YK, Bone ND, Strege AK, Shanafelt TD, Jelinek DF, Kay NE. VEGF receptor phosphorylation status and apoptosis is modulated by a green tea component, epigallocatechin-3-gallate (EGCG), in B-cell chronic lymphocytic leukemia. Blood. 2004;104(3):788-794. - PubMed
-
- Till KJ, Spiller DG, Harris RJ, Chen H, Zuzel M, Cawley JC. CLL, but not normal, B cells are dependent on autocrine VEGF and alpha4beta1 integrin for chemokine-induced motility on and through endothelium. Blood. 2005;105(12):4813-4819. - PubMed
-
- Shanafelt TD, Kay NE. The clinical and biologic importance of neovascularization and angiogenic signaling pathways in chronic lymphocytic leukemia. Semin Oncol. 2006;33(2):174-185. - PubMed

