Population Pharmacokinetic Study of Cefazolin Dosage Adaptation in Bacteremia and Infective Endocarditis Based on a Nomogram
- PMID: 31307987
- PMCID: PMC6761522
- DOI: 10.1128/AAC.00806-19
Population Pharmacokinetic Study of Cefazolin Dosage Adaptation in Bacteremia and Infective Endocarditis Based on a Nomogram
Abstract
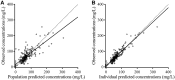
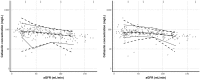
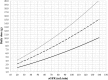
Optimal dosing of continuous-infusion cefazolin can be challenging in patients being treated for bacteremia or infective endocarditis. The aim of this work is to describe and analyze the pharmacokinetics of cefazolin in those patients using a population pharmacokinetics modeling approach and to establish a nomogram to determine the optimal daily dose. Population pharmacokinetics were modeled using the Pmetrics package for R. Plasma concentrations were collected retrospectively from patients treated with continuous-infusion cefazolin for bacteremia or infective endocarditis. The influence of multiple parameters, including renal function, total body weight, body mass index, body surface area (BSA), ideal weight, lean body weight, height, and age, was tested. The probabilities of target attainment for selected target concentrations (40, 60, and 80 mg/liter) were calculated. A dosing nomogram was then developed, using the absolute value of the glomerular filtration rate (aGFR), to determine the optimal daily dose required to achieve the target concentrations in at least 90% of patients. In total, 346 cefazolin plasma concentrations from 162 patients were collected. A one-compartment model best described the data set. The only covariate was aGFR, calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula and the patient's body surface area, for the rate of elimination. Using the nomogram, achieving a cefazolin concentration target of 40 mg/liter with a success rate of at least 90% and with an aGFR of 30, 60, 90, and 120 ml/min requires a daily dose of 2.6, 4.3, 6.1, and 8.0 g/day, respectively. These results confirm the interest of posology adaptation of cefazolin according to aGFR.
Keywords: bacteremia; cefazolin; continuous infusion; infective endocarditis; nonparametric modeling; population pharmacokinetics.
Copyright © 2019 American Society for Microbiology.
Figures
Similar articles
-
Development and validation of a dosing nomogram for continuous infusion cloxacillin in infective endocarditis.J Antimicrob Chemother. 2023 Apr 3;78(4):965-974. doi: 10.1093/jac/dkad030. J Antimicrob Chemother. 2023. PMID: 36760090
-
Development and validation of a dosing nomogram for amoxicillin in infective endocarditis.J Antimicrob Chemother. 2020 Oct 1;75(10):2941-2950. doi: 10.1093/jac/dkaa232. J Antimicrob Chemother. 2020. PMID: 32601687
-
Comparative total and unbound pharmacokinetics of cefazolin administered by bolus versus continuous infusion in patients undergoing major surgery: a randomized controlled trial.Br J Anaesth. 2017 Jun 1;118(6):876-882. doi: 10.1093/bja/aex026. Br J Anaesth. 2017. PMID: 28505360 Clinical Trial.
-
Lack of Pharmacokinetic Basis of Weight-Based Dosing and Intra-Operative Re-Dosing with Cefazolin Surgical Prophylaxis in Obese Patients: Implications for Antibiotic Stewardship.Surg Infect (Larchmt). 2019 Sep;20(6):439-443. doi: 10.1089/sur.2019.039. Epub 2019 May 21. Surg Infect (Larchmt). 2019. PMID: 31112072 Review.
-
Salmonella infective endocarditis.J Microbiol Immunol Infect. 2016 Jun;49(3):313-20. doi: 10.1016/j.jmii.2015.02.659. Epub 2015 Mar 24. J Microbiol Immunol Infect. 2016. PMID: 25882489 Review.
Cited by
-
Comment on: Cannula complications using elastomeric infusers in Hospital in the Home.JAC Antimicrob Resist. 2020 Oct 22;2(4):dlaa088. doi: 10.1093/jacamr/dlaa088. eCollection 2020 Dec. JAC Antimicrob Resist. 2020. PMID: 34223042 Free PMC article. No abstract available.
-
Dosing Cefazolin for Surgical Site Infection Prophylaxis in Adolescent Idiopathic Scoliosis Surgery: Intermittent Bolus or Continuous Infusion?-A Pilot Study.J Clin Med. 2024 Jun 16;13(12):3524. doi: 10.3390/jcm13123524. J Clin Med. 2024. PMID: 38930053 Free PMC article.
-
Nomogram for predicting mortality in hospitalized patients with infective endocarditis.Sci Rep. 2025 Jul 22;15(1):26561. doi: 10.1038/s41598-025-12043-1. Sci Rep. 2025. PMID: 40695963 Free PMC article.
-
Target Attainment and Population Pharmacokinetics of Cefazolin in Patients with Invasive Staphylococcus aureus Infections: A Prospective Cohort Study.Antibiotics (Basel). 2024 Sep 29;13(10):928. doi: 10.3390/antibiotics13100928. Antibiotics (Basel). 2024. PMID: 39452195 Free PMC article.
-
Population Pharmacokinetic and Pharmacodynamic Target Attainment Analysis of Cefazolin in the Serum and Hip Joint Capsule of Patients Undergoing Total Hip Arthroplasty.Antimicrob Agents Chemother. 2021 Mar 18;65(4):e02114-20. doi: 10.1128/AAC.02114-20. Print 2021 Mar 18. Antimicrob Agents Chemother. 2021. PMID: 33526489 Free PMC article.
References
-
- Selton-Suty C, Célard M, Le Moing V, Doco-Lecompte T, Chirouze C, Iung B, Strady C, Revest M, Vandenesch F, Bouvet A, Delahaye F, Alla F, Duval X, Hoen B, AEPEI Study Group. 2012. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis 54:1230–1239. doi:10.1093/cid/cis199. - DOI - PubMed
-
- Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL. 2015. 2015 ESC guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC) Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 36:3075–3128. doi:10.1093/eurheartj/ehv319. - DOI - PubMed
-
- Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O’Gara P, Taubert KA, American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. 2015. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 132:1435–1486. doi:10.1161/CIR.0000000000000296. - DOI - PubMed
-
- McDanel JS, Roghmann M-C, Perencevich EN, Ohl ME, Goto M, Livorsi DJ, Jones M, Albertson JP, Nair R, O’Shea AMJ, Schweizer ML. 2017. Comparative effectiveness of cefazolin versus nafcillin or oxacillin for treatment of methicillin-susceptible Staphylococcus aureus infections complicated by bacteremia: a nationwide cohort study. Clin Infect Dis 65:100–106. doi:10.1093/cid/cix287. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

