Staphylococcus aureus Lipoic Acid Synthesis Limits Macrophage Reactive Oxygen and Nitrogen Species Production To Promote Survival during Infection
- PMID: 31308080
- PMCID: PMC6759302
- DOI: 10.1128/IAI.00344-19
Staphylococcus aureus Lipoic Acid Synthesis Limits Macrophage Reactive Oxygen and Nitrogen Species Production To Promote Survival during Infection
Abstract
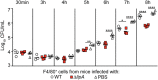
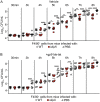
Macrophages are critical mediators of innate immunity and must be overcome for bacterial pathogens to cause disease. The Gram-positive bacterium Staphylococcus aureus produces virulence factors that impede macrophages and other immune cells. We previously determined that production of the metabolic cofactor lipoic acid by the lipoic acid synthetase, LipA, blunts macrophage activation. A ΔlipA mutant was attenuated during infection and was more readily cleared from the host. We hypothesized that bacterial lipoic acid synthesis perturbs macrophage antimicrobial functions and therefore hinders the clearance of S. aureus Here, we found that enhanced innate immune cell activation after infection with a ΔlipA mutant was central to attenuation in vivo, whereas a growth defect imparted by the lipA mutation made a negligible contribution to overall clearance. Macrophages recruited to the site of infection with the ΔlipA mutant produced larger amounts of bactericidal reactive oxygen species (ROS) and reactive nitrogen species (RNS) than those recruited to the site of infection with the wild-type strain or the mutant strain complemented with lipA ROS derived from the NADPH phagocyte oxidase complex and RNS derived from the inducible nitric oxide synthetase, but not mitochondrial ROS, were critical for the restriction of bacterial growth under these conditions. Despite enhanced antimicrobial immunity upon primary infection with the ΔlipA mutant, we found that the host failed to mount an improved recall response to secondary infection. Our data suggest that lipoic acid synthesis in S. aureus promotes bacterial persistence during infection through limitation of ROS and RNS generation by macrophages. Broadly, this work furthers our understanding of the intersections between bacterial metabolism and immune responses to infection.
Keywords: Staphylococcus aureus; lipoic acid; macrophages; metabolism; reactive nitrogen species; reactive oxygen species; virulence.
Copyright © 2019 American Society for Microbiology.
Figures






References
-
- Kourtis AP, Hatfield K, Baggs J, Mu Y, See I, Epson E, Nadle J, Kainer MA, Dumyati G, Petit S, Ray SM, Ham D, Capers C, Ewing H, Coffin N, McDonald LC, Jernigan J, Cardo D. 2019. Vital signs: epidemiology, and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections—United States. MMWR Morb Mortal Wkly Rep 68:214–219. doi:10.15585/mmwr.mm6809e1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

