A Cross-Reactive Protein Vaccine Combined with PCV-13 Prevents Streptococcus pneumoniae- and Haemophilus influenzae-Mediated Acute Otitis Media
- PMID: 31308088
- PMCID: PMC6759292
- DOI: 10.1128/IAI.00253-19
A Cross-Reactive Protein Vaccine Combined with PCV-13 Prevents Streptococcus pneumoniae- and Haemophilus influenzae-Mediated Acute Otitis Media
Abstract
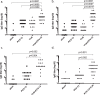
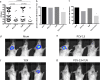
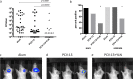
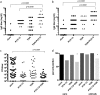
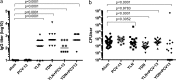
Acute otitis media is one of the most common childhood infections worldwide. Currently licensed vaccines against the common otopathogen Streptococcus pneumoniae target the bacterial capsular polysaccharide and confer no protection against nonencapsulated strains or capsular types outside vaccine coverage. Mucosal infections such as acute otitis media remain prevalent, even those caused by vaccine-covered serotypes. Here, we report that a protein-based vaccine, a fusion construct of epitopes of CbpA to pneumolysin toxoid, confers effective protection against pneumococcal acute otitis media for non-PCV-13 serotypes and enhances protection for PCV-13 serotypes when coadministered with PCV-13. Having cross-reactive epitopes, the fusion protein also induces potent antibody responses against nontypeable Haemophilus influenzae and S. pneumoniae, engendering protection against acute otitis media caused by emerging unencapsulated otopathogens. These data suggest that augmenting capsule-based vaccination with conserved, cross-reactive protein-based vaccines broadens and enhances protection against acute otitis media.
Keywords: Haemophilus influenzae, Streptococcus pneumoniae; otitis media; vaccines.
Copyright © 2019 American Society for Microbiology.
Figures
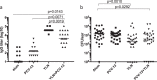
Similar articles
-
Broadly protective protein-based pneumococcal vaccine composed of pneumolysin toxoid-CbpA peptide recombinant fusion protein.J Infect Dis. 2014 Apr 1;209(7):1116-25. doi: 10.1093/infdis/jit502. Epub 2013 Sep 16. J Infect Dis. 2014. PMID: 24041791 Free PMC article.
-
Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study.Lancet. 2006 Mar 4;367(9512):740-8. doi: 10.1016/S0140-6736(06)68304-9. Lancet. 2006. PMID: 16517274 Clinical Trial.
-
Impact of protein D-containing pneumococcal conjugate vaccines on non-typeable Haemophilus influenzae acute otitis media and carriage.Expert Rev Vaccines. 2017 Jul;16(7):1-14. doi: 10.1080/14760584.2017.1333905. Epub 2017 Jun 7. Expert Rev Vaccines. 2017. PMID: 28571504 Review.
-
Efficacy, safety and immunogenicity of a pneumococcal protein-based vaccine co-administered with 13-valent pneumococcal conjugate vaccine against acute otitis media in young children: A phase IIb randomized study.Vaccine. 2019 Dec 3;37(51):7482-7492. doi: 10.1016/j.vaccine.2019.09.076. Epub 2019 Oct 16. Vaccine. 2019. PMID: 31629570 Clinical Trial.
-
Otitis media: prospects for prevention.Vaccine. 2008 Dec 23;26 Suppl 7:G20-4. doi: 10.1016/j.vaccine.2008.11.009. Vaccine. 2008. PMID: 19094934 Review.
Cited by
-
Perspective of a Pediatrician: Shared Pathogenesis of the Three Most Successful Pathogens of Children.Front Cell Infect Microbiol. 2020 Oct 15;10:585791. doi: 10.3389/fcimb.2020.585791. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33178633 Free PMC article.
-
Panel 1: Biotechnology, biomedical engineering and new models of otitis media.Int J Pediatr Otorhinolaryngol. 2020 Mar;130 Suppl 1(Suppl 1):109833. doi: 10.1016/j.ijporl.2019.109833. Epub 2019 Dec 27. Int J Pediatr Otorhinolaryngol. 2020. PMID: 31901291 Free PMC article. Review.
-
Opening the OPK Assay Gatekeeper: Harnessing Multi-Modal Protection by Pneumococcal Vaccines.Pathogens. 2019 Oct 23;8(4):203. doi: 10.3390/pathogens8040203. Pathogens. 2019. PMID: 31652741 Free PMC article.
-
A Retrospective Database Analysis to Estimate the Burden of Acute Otitis Media in Children Aged <15 Years in the Veneto Region (Italy).Children (Basel). 2022 Mar 19;9(3):436. doi: 10.3390/children9030436. Children (Basel). 2022. PMID: 35327808 Free PMC article.
-
A Trivalent Live Vaccine Elicits Cross-Species Protection Against Acute Otitis Media in a Murine Model.Vaccines (Basel). 2024 Dec 19;12(12):1432. doi: 10.3390/vaccines12121432. Vaccines (Basel). 2024. PMID: 39772092 Free PMC article.
References
-
- Livorsi DJ, Macneil JR, Cohn AC, Bareta J, Zansky S, Petit S, Gershman K, Harrison LH, Lynfield R, Reingold A, Schaffner W, Thomas A, Farley MM. 2012. Invasive Haemophilus influenzae in the United States, 1999–2008: epidemiology and outcomes. J Infect 65:496–504. doi:10.1016/j.jinf.2012.08.005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

