Control of Tissue-Resident Invariant NKT Cells by Vitamin A Metabolites and P2X7-Mediated Cell Death
- PMID: 31308092
- PMCID: PMC6730561
- DOI: 10.4049/jimmunol.1900398
Control of Tissue-Resident Invariant NKT Cells by Vitamin A Metabolites and P2X7-Mediated Cell Death
Abstract
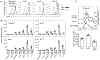
Invariant NKT (iNKT) cells provide rapid innate T cell responses to glycolipid Ags from host cells and microbes. The numbers of CD1d-restricted iNKT cells are tightly controlled in mucosal tissues, but the mechanisms have been largely unclear. We found that vitamin A is a dominant factor that controls the population size of mucosal iNKT cells in mice. This negative regulation is mediated by the induction of the purinergic receptor P2X7 on iNKT cells. The expression of P2X7 is particularly high on intestinal iNKT cells, making iNKT cells highly susceptible to P2X7-mediated cell death. In vitamin A deficiency, iNKT cells fail to express P2X7 and are, therefore, resistant to P2X7-mediated cell death, leading to iNKT cell overpopulation. This phenomenon is most prominent in the intestine. We found that iNKT cells are divided into CD69+ sphingosine-1-phosphate receptor 1 (S1P1)- tissue resident and CD69- S1P1+ nonresident iNKT cells. The CD69+ S1P1- tissue-resident iNKT cells highly express P2X7 and are effectively controlled by the P2X7 pathway. The regulation of iNKT cells by vitamin A by the P2X7 pathway is important to prevent aberrant expansion of effector cytokine-producing iNKT cells. Our findings identify a novel role of vitamin A in regulating iNKT cell homeostasis in many tissues throughout the body.
Copyright © 2019 by The American Association of Immunologists, Inc.
Conflict of interest statement
Figures




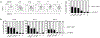


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

