Early Life Inflammation Increases CA1 Pyramidal Neuron Excitability in a Sex and Age Dependent Manner through a Chloride Homeostasis Disruption
- PMID: 31308096
- PMCID: PMC6759023
- DOI: 10.1523/JNEUROSCI.2973-18.2019
Early Life Inflammation Increases CA1 Pyramidal Neuron Excitability in a Sex and Age Dependent Manner through a Chloride Homeostasis Disruption
Abstract
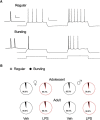
Early life, systemic inflammation causes long-lasting changes in behavior. To unmask possible mechanisms associated with this phenomenon, we asked whether the intrinsic membrane properties in hippocampal neurons were altered as a consequence of early life inflammation. C57BL/6 mice were bred in-house and both male and female pups from multiple litters were injected with lipopolysaccharide (LPS; 100 μg/kg, i.p.) or vehicle at postnatal day (P)14, and kept until adolescence (P35-P45) or adulthood (P60-P70), when brain slices were prepared for whole-cell and perforated-patch recordings from CA1 hippocampal pyramidal neurons. In neurons of adult male mice pretreated with LPS, the number of action potentials elicited by depolarizing current pulses was significantly increased compared with control neurons, concomitant with increased input resistance, and a lower action potential threshold. Although these changes were not associated with changes in relevant sodium channel expression or differences in capacitance or dendritic architecture, they were linked to a mechanism involving intracellular chloride overload, revealed through a depolarized GABA reversal potential and increased expression of the chloride transporter, NKCC1. In contrast, no significant changes were observed in neurons of adult female mice pretreated with LPS, nor in adolescent mice of either sex. These data uncover a potential mechanism involving neonatal inflammation-induced plasticity in chloride homeostasis, which may contribute to early life inflammation-induced behavioral alterations.SIGNIFICANCE STATEMENT Early life inflammation results in long-lasting changes in many aspects of adult physiology. In this paper we reveal that a brief exposure to early life peripheral inflammation with LPS increases excitability in hippocampal neurons in a sex- and age-dependent manner through a chloride homeostasis disruption. As this hyperexcitability was only seen in adult males, and not in adult females or adolescent animals of either sex, it raises the possibility of a hormonal interaction with early life inflammation. Furthermore, as neonatal inflammation is a normal feature of early life in most animals, as well as humans, these findings may be very important for the development of animal models of disease that more appropriately resemble the human condition.
Keywords: GABA reversal potential; hippocampus; inflammation; intrinsic membrane properties; lipopolysaccharide; sex differences.
Copyright © 2019 the authors.
Figures








Similar articles
-
Increased Excitatory Synaptic Transmission Associated with Adult Seizure Vulnerability Induced by Early-Life Inflammation in Mice.J Neurosci. 2021 May 19;41(20):4367-4377. doi: 10.1523/JNEUROSCI.2667-20.2021. Epub 2021 Apr 7. J Neurosci. 2021. PMID: 33827934 Free PMC article.
-
Chronic stress shifts the GABA reversal potential in the hippocampus and increases seizure susceptibility.Epilepsy Res. 2015 Jan;109:13-27. doi: 10.1016/j.eplepsyres.2014.10.003. Epub 2014 Oct 23. Epilepsy Res. 2015. PMID: 25524838 Free PMC article.
-
Chronic LPS exposure produces changes in intrinsic membrane properties and a sustained IL-beta-dependent increase in GABAergic inhibition in hippocampal CA1 pyramidal neurons.Hippocampus. 2005;15(5):656-64. doi: 10.1002/hipo.20086. Hippocampus. 2005. PMID: 15889405
-
Hyperexcitability of hippocampal CA1 pyramidal neurons in male offspring of a rat model of autism spectrum disorder (ASD) induced by prenatal exposure to valproic acid: A possible involvement of Ih channel current.Brain Res. 2019 Apr 1;1708:188-199. doi: 10.1016/j.brainres.2018.12.011. Epub 2018 Dec 8. Brain Res. 2019. PMID: 30537517
-
Synergistic Positive Feedback Mechanisms Underlying Seizure Initiation.Epilepsy Curr. 2022 Sep 27;23(1):38-43. doi: 10.1177/15357597221127163. eCollection 2023 Jan-Feb. Epilepsy Curr. 2022. PMID: 36923333 Free PMC article. Review.
Cited by
-
Inhibition of Connexin 36 attenuates HMGB1-mediated depressive-like behaviors induced by chronic unpredictable mild stress.Brain Behav. 2022 Feb;12(2):e2470. doi: 10.1002/brb3.2470. Epub 2022 Jan 28. Brain Behav. 2022. PMID: 35089644 Free PMC article.
-
Magnetic Resonance Imaging Correlates of White Matter Gliosis and Injury in Preterm Fetal Sheep Exposed to Progressive Systemic Inflammation.Int J Mol Sci. 2020 Nov 24;21(23):8891. doi: 10.3390/ijms21238891. Int J Mol Sci. 2020. PMID: 33255257 Free PMC article.
-
Inhibition of the NKCC1/NF-κB Signaling Pathway Decreases Inflammation and Improves Brain Edema and Nerve Cell Apoptosis in an SBI Rat Model.Front Mol Neurosci. 2021 Mar 31;14:641993. doi: 10.3389/fnmol.2021.641993. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33867933 Free PMC article.
-
Maternal antibiotic administration during gestation can affect the memory and brain structure in mouse offspring.Front Cell Neurosci. 2023 May 10;17:1176676. doi: 10.3389/fncel.2023.1176676. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37234915 Free PMC article.
-
Severe inflammation in new-borns induces long-term cognitive impairment by activation of IL-1β/KCC2 signaling during early development.BMC Med. 2022 Jul 27;20(1):235. doi: 10.1186/s12916-022-02434-w. BMC Med. 2022. PMID: 35883093 Free PMC article.
References
-
- Acharjee S, Verbeek M, Gomez CD, Bisht K, Lee B, Benoit L, Sharkey KA, Benediktsson A, Tremblay ME, Pittman QJ (2018) Reduced microglial activity and enhanced glutamate transmission in the basolateral amygdala in early CNS autoimmunity. J Neurosci 38:9019–9033. 10.1523/JNEUROSCI.0398-18.2018 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
