Targeting Seizure-Induced Neurogenesis in a Clinically Relevant Time Period Leads to Transient But Not Persistent Seizure Reduction
- PMID: 31308098
- PMCID: PMC6733567
- DOI: 10.1523/JNEUROSCI.0920-19.2019
Targeting Seizure-Induced Neurogenesis in a Clinically Relevant Time Period Leads to Transient But Not Persistent Seizure Reduction
Abstract
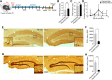
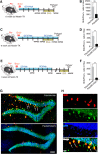
Mesial temporal lobe epilepsy (mTLE), the most common form of medically refractory epilepsy in adults, is usually associated with hippocampal pathophysiology. Using rodent models of mTLE, many studies including work from our laboratory have shown that new neurons born around the onset of severe acute seizures known as status epilepticus (SE) are crucial for the process of epileptogenesis and targeting seizure-induced neurogenesis either genetically or pharmacologically can impact the frequency of chronic seizures. However, these studies are limited in their clinical relevance as none of them determines the potential of blocking new neurons generated after the epileptogenic insult to alleviate the development of chronic seizures. Therefore, using a pilocarpine-induced SE model of mTLE in mice of either sex, we show that >4 weeks of continuous and concurrent ablation of seizure-induced neurogenesis after SE can reduce the formation of spontaneous recurrent seizures by 65%. We also found that blocking post-SE neurogenesis does not lead to long-term seizure reduction as the effect was observed only transiently for 10 d with >4 weeks of continuous and concurrent ablation of seizure-induced neurogenesis. Thus, these findings provide evidence that seizure-induced neurogenesis when adequately reduced in a clinically relevant time period has the potential to transiently suppress recurrent seizures, but additional mechanisms need to be targeted to permanently prevent epilepsy development.SIGNIFICANCE STATEMENT Consistent with morphological and electrophysiological studies suggesting aberrant adult-generated neurons contribute to epilepsy development, ablation of seizure-induced new neurons at the time of the initial insult reduces the frequency of recurrent seizures. In this study, we show that continuous targeting of post-insult new neurons in a therapeutically relevant time period reduces chronic seizures; however, this effect does not persist suggesting possible additional mechanisms.
Keywords: adult neurogenesis; epilepsy; hippocampus; mTLE; neural stem cell; newborn neurons.
Copyright © 2019 the authors.
Figures



Similar articles
-
Circadian clustering of spontaneous epileptic seizures emerges after pilocarpine-induced status epilepticus.Epilepsia. 2017 Jul;58(7):1159-1171. doi: 10.1111/epi.13795. Epub 2017 May 24. Epilepsia. 2017. PMID: 28542864
-
Roles of astrocytes and microglia in seizure-induced aberrant neurogenesis in the hippocampus of adult rats.J Neurosci Res. 2010 Feb 15;88(3):519-29. doi: 10.1002/jnr.22224. J Neurosci Res. 2010. PMID: 19774666
-
The polarity and properties of radial glia-like neural stem cells are altered by seizures with status epilepticus: Study using an improved mouse pilocarpine model of epilepsy.Hippocampus. 2020 Mar;30(3):250-262. doi: 10.1002/hipo.23153. Epub 2019 Sep 5. Hippocampus. 2020. PMID: 32101365
-
Mechanisms and functional significance of aberrant seizure-induced hippocampal neurogenesis.Epilepsia. 2008 Jun;49 Suppl 5:19-25. doi: 10.1111/j.1528-1167.2008.01634.x. Epilepsia. 2008. PMID: 18522597 Review.
-
Seizure-induced neurogenesis: are more new neurons good for an adult brain?Prog Brain Res. 2002;135:121-31. doi: 10.1016/S0079-6123(02)35012-X. Prog Brain Res. 2002. PMID: 12143334 Review.
Cited by
-
Rise and Fall of the Empire: Conquering Alzheimer's Disease by Targeting Adult Neurogenesis.Epilepsy Curr. 2019 Nov-Dec;19(6):411-413. doi: 10.1177/1535759719874813. Epub 2019 Sep 16. Epilepsy Curr. 2019. PMID: 31526033 Free PMC article.
-
From single-neuron dynamics to higher-order circuit motifs in control and pathological brain networks.J Physiol. 2023 Aug;601(15):3011-3024. doi: 10.1113/JP282749. Epub 2022 Jul 22. J Physiol. 2023. PMID: 35815823 Free PMC article.
-
HOP protein expression in the hippocampal dentate gyrus is acutely downregulated in a status epilepticus mouse model.IBRO Neurosci Rep. 2021 Oct 23;11:183-193. doi: 10.1016/j.ibneur.2021.10.002. eCollection 2021 Dec. IBRO Neurosci Rep. 2021. PMID: 34766103 Free PMC article.
-
Hippocampal adult-born granule cells drive network activity in a mouse model of chronic temporal lobe epilepsy.Nat Commun. 2020 Dec 1;11(1):6138. doi: 10.1038/s41467-020-19969-2. Nat Commun. 2020. PMID: 33262339 Free PMC article.
-
Hippocampal transplants of fetal GABAergic progenitors regulate adult neurogenesis in mice with temporal lobe epilepsy.Neurobiol Dis. 2022 Nov;174:105879. doi: 10.1016/j.nbd.2022.105879. Epub 2022 Sep 29. Neurobiol Dis. 2022. PMID: 36183946 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
