Calcium Signaling in Cardiomyocyte Function
- PMID: 31308143
- PMCID: PMC7050587
- DOI: 10.1101/cshperspect.a035428
Calcium Signaling in Cardiomyocyte Function
Abstract
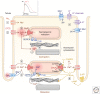
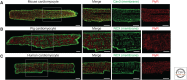
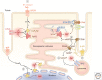
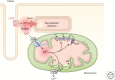
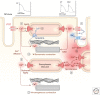
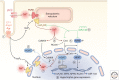
Rhythmic increases in intracellular Ca2+ concentration underlie the contractile function of the heart. These heart muscle-wide changes in intracellular Ca2+ are induced and coordinated by electrical depolarization of the cardiomyocyte sarcolemma by the action potential. Originating at the sinoatrial node, conduction of this electrical signal throughout the heart ensures synchronization of individual myocytes into an effective cardiac pump. Ca2+ signaling pathways also regulate gene expression and cardiomyocyte growth during development and in pathology. These fundamental roles of Ca2+ in the heart are illustrated by the prevalence of altered Ca2+ homeostasis in cardiovascular diseases. Indeed, heart failure (an inability of the heart to support hemodynamic needs), rhythmic disturbances, and inappropriate cardiac growth all share an involvement of altered Ca2+ handling. The prevalence of these pathologies, contributing to a third of all deaths in the developed world as well as to substantial morbidity makes understanding the mechanisms of Ca2+ handling and dysregulation in cardiomyocytes of great importance.
Copyright © 2020 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
Similar articles
-
ß-blocker timolol prevents arrhythmogenic Ca²⁺ release and normalizes Ca²⁺ and Zn²⁺ dyshomeostasis in hyperglycemic rat heart.PLoS One. 2013 Jul 29;8(7):e71014. doi: 10.1371/journal.pone.0071014. Print 2013. PLoS One. 2013. PMID: 23923043 Free PMC article.
-
Phospholamban ablation in hearts expressing the high affinity SERCA2b isoform normalizes global Ca²⁺ homeostasis but not Ca²⁺-dependent hypertrophic signaling.Am J Physiol Heart Circ Physiol. 2012 Jun 15;302(12):H2574-82. doi: 10.1152/ajpheart.01166.2011. Epub 2012 Apr 13. Am J Physiol Heart Circ Physiol. 2012. PMID: 22505640
-
Role of the calcium-sensing receptor in cardiomyocyte apoptosis via the sarcoplasmic reticulum and mitochondrial death pathway in cardiac hypertrophy and heart failure.Cell Physiol Biochem. 2013;31(4-5):728-43. doi: 10.1159/000350091. Epub 2013 May 23. Cell Physiol Biochem. 2013. PMID: 23711498
-
Calcium-regulated transcriptional pathways in the normal and pathologic heart.IUBMB Life. 2011 Oct;63(10):847-55. doi: 10.1002/iub.545. Epub 2011 Sep 7. IUBMB Life. 2011. PMID: 21901815 Review.
-
Unique Ca2+-Cycling Protein Abundance and Regulation Sustains Local Ca2+ Releases and Spontaneous Firing of Rabbit Sinoatrial Node Cells.Int J Mol Sci. 2018 Jul 25;19(8):2173. doi: 10.3390/ijms19082173. Int J Mol Sci. 2018. PMID: 30044420 Free PMC article. Review.
Cited by
-
Cardiomyocyte adhesion and hyperadhesion differentially require ERK1/2 and plakoglobin.JCI Insight. 2020 Sep 17;5(18):e140066. doi: 10.1172/jci.insight.140066. JCI Insight. 2020. PMID: 32841221 Free PMC article.
-
Fundamentals of Cellular Calcium Signaling: A Primer.Cold Spring Harb Perspect Biol. 2020 Jan 2;12(1):a038802. doi: 10.1101/cshperspect.a038802. Cold Spring Harb Perspect Biol. 2020. PMID: 31427372 Free PMC article. Review.
-
Sertraline-induced 5-HT dysregulation in mouse cardiomyocytes and the impact on calcium handling.Am J Physiol Heart Circ Physiol. 2024 Dec 1;327(6):H1559-H1576. doi: 10.1152/ajpheart.00692.2023. Epub 2024 Oct 18. Am J Physiol Heart Circ Physiol. 2024. PMID: 39423037 Free PMC article.
-
Embryo Development in a Stochastic Universe.Bioelectricity. 2024 Sep 16;6(3):196-203. doi: 10.1089/bioe.2023.0050. eCollection 2024 Sep. Bioelectricity. 2024. PMID: 39372089
-
Cardiac function, structural, and electrical remodeling by microgravity exposure.Am J Physiol Heart Circ Physiol. 2023 Jan 1;324(1):H1-H13. doi: 10.1152/ajpheart.00611.2022. Epub 2022 Nov 18. Am J Physiol Heart Circ Physiol. 2023. PMID: 36399385 Free PMC article. Review.
References
-
- Arantes LA, Aguiar CJ, Amaya MJ, Figueiró NC, Andrade LM, Rocha-Resende C, Resende RR, Franchini KG, Guatimosim S, Leite MF. 2012. Nuclear inositol 1,4,5-trisphosphate is a necessary and conserved signal for the induction of both pathological and physiological cardiomyocyte hypertrophy. J Mol Cell Cardiol 53: 475–486. 10.1016/j.yjmcc.2012.06.017 - DOI - PubMed
-
- Avila-Medina J, Mayoral-Gonzalez I, Dominguez-Rodriguez A, Gallardo-Castillo I, Ribas J, Ordoñez A, Rosado JA, Smani T. 2018. The complex role of store operated calcium entry pathways and related proteins in the function of cardiac, skeletal and vascular smooth muscle cells. Front Physiol 9: 257 10.3389/fphys.2018.00257 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
