Effect of Pregnancy on Paroxetine-Induced Adiposity and Glucose Intolerance in Mice
- PMID: 31308195
- PMCID: PMC6750187
- DOI: 10.1124/jpet.118.255380
Effect of Pregnancy on Paroxetine-Induced Adiposity and Glucose Intolerance in Mice
Abstract
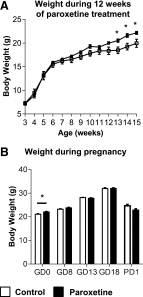
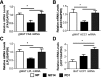
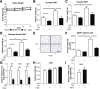
Long-term use of selective serotonin reuptake inhibitors (SSRIs) targeting the serotonin transporter (SERT) has been suggested to be associated with an increased risk for obesity and type 2 diabetes. Previously, using a murine knockout model of SERT, we showed that estrogen suppression is involved in SERT deficiency-induced obesity and glucose intolerance in nonpregnant mice. The present study investigated the effects of chronic paroxetine treatment on adiposity and glucose tolerance in mice before and during pregnancy. Chronic paroxetine treatment in nonpregnant mice resulted in visceral adiposity and glucose intolerance accompanied by reduced circulating 17β-estradiol levels and ovarian expression of the aromatase (CYP19a1). Remarkably, pregnancy significantly reduced adiposity and improved glucose tolerance in paroxetine-treated mice by rebooting ovarian CYP19a1 expression and 17β-estradiol production. These effects appear to be reversible as ovarian CYP19a1 expression and circulating 17β-estradiol returned to prepregnancy levels soon after parturition. As in pregnant mice, 17β-estradiol replacement treatment in nonpregnant mice reduced paroxetine-induced adiposity. Our findings further suggested that modulation of estrogen synthesis underlies the observed metabolic adverse effects of SSRIs. Although our data revealed a transient reversal effect of pregnancy on SSRI-induced metabolic abnormalities, these observations are experimental and limited to mice. The use of SSRIs during human pregnancy should be cautioned because of potential adverse effects to the fetuses.
Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



Similar articles
-
Serotonin transporter deficiency drives estrogen-dependent obesity and glucose intolerance.Sci Rep. 2017 Apr 25;7(1):1137. doi: 10.1038/s41598-017-01291-5. Sci Rep. 2017. PMID: 28442777 Free PMC article.
-
Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population-based cohort study from the Netherlands.Br J Clin Pharmacol. 2008 Apr;65(4):600-6. doi: 10.1111/j.1365-2125.2007.03048.x. Epub 2007 Oct 22. Br J Clin Pharmacol. 2008. PMID: 17953715 Free PMC article.
-
[Treatment of depressed pregnant women by selective serotonin reuptake inhibitors: risk for the foetus and the newborn].Encephale. 2010 Jun;36 Suppl 2:D133-8. doi: 10.1016/j.encep.2009.06.005. Epub 2009 Sep 19. Encephale. 2010. PMID: 20513456 Review. French.
-
Relationship between brain serotonin transporter binding, plasma concentration and behavioural effect of selective serotonin reuptake inhibitors.Br J Pharmacol. 2005 Mar;144(5):695-702. doi: 10.1038/sj.bjp.0706108. Br J Pharmacol. 2005. PMID: 15678084 Free PMC article.
-
Selective serotonin reuptake inhibitor exposure during early pregnancy and the risk of fetal major malformations: focus on paroxetine.J Clin Psychiatry. 2009 Mar;70(3):414-22. doi: 10.4088/jcp.08r04468. Epub 2009 Feb 24. J Clin Psychiatry. 2009. PMID: 19254517 Review.
Cited by
-
Impact of Chronic Fluoxetine Exposure on Oocyte Development and Reproductive Outcomes in a Mouse Model.Int J Mol Sci. 2025 May 19;26(10):4858. doi: 10.3390/ijms26104858. Int J Mol Sci. 2025. PMID: 40429999 Free PMC article.
-
Uptake and Metabolization of Serotonin by Granulosa Cells Form a Functional Barrier in the Mouse Ovary.Int J Mol Sci. 2022 Nov 27;23(23):14828. doi: 10.3390/ijms232314828. Int J Mol Sci. 2022. PMID: 36499156 Free PMC article.
-
Continuous home cage monitoring of activity and sleep in mice during repeated paroxetine treatment and discontinuation.Psychopharmacology (Berl). 2023 Nov;240(11):2403-2418. doi: 10.1007/s00213-023-06442-3. Epub 2023 Aug 16. Psychopharmacology (Berl). 2023. PMID: 37584734 Free PMC article.
-
Longitudinal metabolic profiling of women using selective serotonin reuptake inhibitors during pregnancy.Metabolomics. 2025 Aug 21;21(5):123. doi: 10.1007/s11306-025-02334-z. Metabolomics. 2025. PMID: 40839053 Free PMC article.
-
Serotonin transporter-deficient mice display enhanced adipose tissue inflammation after chronic high-fat diet feeding.Front Immunol. 2023 Jul 13;14:1184010. doi: 10.3389/fimmu.2023.1184010. eCollection 2023. Front Immunol. 2023. PMID: 37520561 Free PMC article.
References
-
- Al-Qahtani SM, Bryzgalova G, Valladolid-Acebes I, Korach-André M, Dahlman-Wright K, Efendić S, Berggren PO, Portwood N. (2017) 17β-Estradiol suppresses visceral adipogenesis and activates brown adipose tissue-specific gene expression. Horm Mol Biol Clin Investig 29:13–26. - PubMed
-
- Alwan S, Friedman JM. (2009) Safety of selective serotonin reuptake inhibitors in pregnancy. CNS Drugs 23:493–509. - PubMed
-
- Andersohn F, Schade R, Suissa S, Garbe E. (2009) Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry 166:591–598. - PubMed
-
- Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, Rolnick SJ, Roblin D, Smith DH, Willy ME, et al. (2008) Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol 198:194.e1-e5. - PubMed
-
- Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. (2002) betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297:843–845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

