An immunometabolic pathomechanism for chronic obstructive pulmonary disease
- PMID: 31308239
- PMCID: PMC6681742
- DOI: 10.1073/pnas.1906303116
An immunometabolic pathomechanism for chronic obstructive pulmonary disease
Abstract
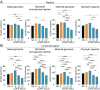
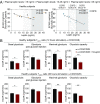
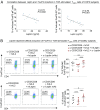
Chronic obstructive pulmonary disease (COPD) is an inflammatory condition associated with abnormal immune responses, leading to airflow obstruction. Lungs of COPD subjects show accumulation of proinflammatory T helper (Th) 1 and Th17 cells resembling that of autoreactive immune responses. As regulatory T (Treg) cells play a central role in the control of autoimmune responses and their generation and function are controlled by the adipocytokine leptin, we herein investigated the association among systemic leptin overproduction, reduced engagement of glycolysis in T cells, and reduced peripheral frequency of Treg cells in different COPD stages. These phenomena were also associated with an impaired capacity to generate inducible Treg (iTreg) cells from conventional T (Tconv) cells. At the molecular level, we found that leptin inhibited the expression of forkhead-boxP3 (FoxP3) and its splicing variants containing the exon 2 (FoxP3-E2) that correlated inversely with inflammation and weakened lung function during COPD progression. Our data reveal that the immunometabolic pathomechanism leading to COPD progression is characterized by leptin overproduction, a decline in the expression of FoxP3 splicing forms, and an impairment in Treg cell generation and function. These results have potential implications for better understanding the autoimmune-like nature of COPD and the pathogenic events leading to lung damage.
Keywords: COPD; immunometabolism; leptin; regulatory T cells.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Barnes P. J., et al. , Chronic obstructive pulmonary disease. Nat. Rev. Dis. Primers 1, 15076 (2015). - PubMed
-
- Cosio M. G., Saetta M., Agusti A., Immunologic aspects of chronic obstructive pulmonary disease. N. Engl. J. Med. 360, 2445–2454 (2009). - PubMed
-
- Rahman I., Adcock I. M., Oxidative stress and redox regulation of lung inflammation in COPD. Eur. Respir. J. 28, 219–242 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

