The Kindlin2-p53-SerpinB2 signaling axis is required for cellular senescence in breast cancer
- PMID: 31308359
- PMCID: PMC6629707
- DOI: 10.1038/s41419-019-1774-z
The Kindlin2-p53-SerpinB2 signaling axis is required for cellular senescence in breast cancer
Abstract
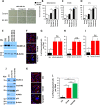
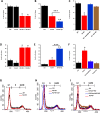
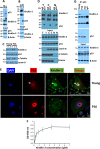
In cancer, cellular senescence is a complex process that leads to inhibition of proliferation of cells that may develop a neoplastic phenotype. A plethora of signaling pathways, when dysregulated, have been shown to elicit a senescence response. Two well-known tumor suppressor pathways, controlled by the p53 and retinoblastoma proteins, have been implicated in maintaining the cellular senescence phenotype. Kindlin-2, a member of an actin cytoskeleton organizing and integrin activator proteins, has been shown to play a key role in the regulation of several hallmarks of several cancers, including breast cancer (BC). The molecular mechanisms whereby Kindlin-2 regulates cellular senescence in BC tumors remains largely unknown. Here we show that Kindlin-2 regulates cellular senescence in part through its interaction with p53, whereby it regulates the expression of the p53-responsive genes; i.e., SerpinB2 and p21, during the induction of senescence. Our data show that knockout of Kindlin-2 via CRISPR/Cas9 in several BC cell lines significantly increases expression levels of both SerpinB2 and p21 resulting in the activation of hallmarks of cellular senescence. Mechanistically, interaction between Kindlin-2 and p53 at the promotor level is critical for the regulated expression of SerpinB2 and p21. These findings identify a previously unknown Kindlin-2/p53/SerpinB2 signaling axis that regulates cellular senescence and intervention in this axis may serve as a new therapeutic window for BCs treatment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

