Enantioselective construction of remote tertiary carbon-fluorine bonds
- PMID: 31308495
- PMCID: PMC6679931
- DOI: 10.1038/s41557-019-0289-7
Enantioselective construction of remote tertiary carbon-fluorine bonds
Abstract
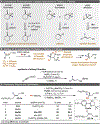
The carbon-fluorine bond engenders distinctive physicochemical properties and significant changes to general reactivity. The development of catalytic, enantioselective methods to set stereocentres that contain a benzylic C-F bond is a rapidly evolving goal in synthetic chemistry. Although there have been notable advances that enable the construction of secondary stereocentres that contain both a C-F and a C-H bond on the same carbon, significantly fewer strategies are defined to access stereocentres that incorporate a tertiary C-F bond, especially those remote from pre-existing activating groups. Here we report a general method that establishes C-F tertiary benzylic stereocentres by forging a C-C bond via a Pd-catalysed enantioselective Heck reaction of acyclic alkenyl fluorides with arylboronic acids. This method provides a platform to rapidly incorporate significant functionality about the benzylic tertiary fluoride by virtue of the diversity of both reaction partners, as well as the ability to install the stereocentres remotely from pre-existing functional groups.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Enantioselective construction of remote quaternary stereocentres.Nature. 2014 Apr 17;508(7496):340-4. doi: 10.1038/nature13231. Epub 2014 Apr 9. Nature. 2014. PMID: 24717439 Free PMC article.
-
Desymmetrization of difluoromethylene groups by C-F bond activation.Nature. 2020 Jul;583(7817):548-553. doi: 10.1038/s41586-020-2399-1. Epub 2020 Jun 1. Nature. 2020. PMID: 32480398 Free PMC article.
-
Copper-Catalyzed Radical Relay for Asymmetric Radical Transformations.Acc Chem Res. 2018 Sep 18;51(9):2036-2046. doi: 10.1021/acs.accounts.8b00265. Epub 2018 Sep 5. Acc Chem Res. 2018. PMID: 30183262
-
The organometallic fluorine chemistry of palladium and rhodium: studies toward aromatic fluorination.Acc Chem Res. 2010 Jan 19;43(1):160-71. doi: 10.1021/ar9001763. Acc Chem Res. 2010. PMID: 19788304 Review.
-
Recent Advances in Transition-metal-catalyzed C-C Bond Formation via C(sp2 )-F Bond Cleavage.Chem Rec. 2021 Dec;21(12):3394-3410. doi: 10.1002/tcr.202100053. Epub 2021 Apr 14. Chem Rec. 2021. PMID: 33852203 Review.
Cited by
-
Enantioselective Construction of Tertiary Fluoride Stereocenters by Organocatalytic Fluorocyclization.J Am Chem Soc. 2020 Nov 25;142(47):20048-20057. doi: 10.1021/jacs.0c09323. Epub 2020 Nov 16. J Am Chem Soc. 2020. PMID: 33191747 Free PMC article.
-
Facile construction of distal and diversified tertiary and quaternary stereocenters.Proc Natl Acad Sci U S A. 2024 Dec 17;121(51):e2408541121. doi: 10.1073/pnas.2408541121. Epub 2024 Dec 12. Proc Natl Acad Sci U S A. 2024. PMID: 39665763 Free PMC article.
-
Asymmetric synthesis of γ-chiral borylalkanes via sequential reduction/hydroboration using a single copper catalyst.Chem Sci. 2020 Aug 6;11(33):8961-8965. doi: 10.1039/d0sc03759a. Chem Sci. 2020. PMID: 34123150 Free PMC article.
-
Development and Mechanistic Interrogation of Interrupted Chain-Walking in the Enantioselective Relay Heck Reaction.J Am Chem Soc. 2020 Jun 10;142(23):10516-10525. doi: 10.1021/jacs.0c03589. Epub 2020 May 29. J Am Chem Soc. 2020. PMID: 32412759 Free PMC article.
-
Copper-Catalyzed Defluorinative Borylation and Silylation of gem-Difluoroallyl Groups.Org Lett. 2020 Sep 4;22(17):6805-6809. doi: 10.1021/acs.orglett.0c02321. Epub 2020 Aug 25. Org Lett. 2020. PMID: 32841569 Free PMC article.
References
-
- Brunet VA & O’Hagan D Catalytic asymmetric fluorination comes of age. Angew. Chem. Int. Ed 47, 1179–1182 (2008). - PubMed
-
- Ma J-A & Cahard D Update 1 of: asymmetric fluorination, trifluoromethylation, and perfluoroalkylation reactions. Chem. Rev 108, PR1–PR43 (2008). - PubMed
-
- Differding E & Lang RW New fluorinating reagents-I. the first enantioselec-tive fluorination reaction. Tetrahedron Lett. 29, 6087–6090 (1988).

