Highly rearranged chromosomes reveal uncoupling between genome topology and gene expression
- PMID: 31308546
- PMCID: PMC7116017
- DOI: 10.1038/s41588-019-0462-3
Highly rearranged chromosomes reveal uncoupling between genome topology and gene expression
Abstract
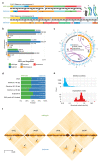
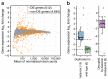
Chromatin topology is intricately linked to gene expression, yet its functional requirement remains unclear. Here, we comprehensively assessed the interplay between genome topology and gene expression using highly rearranged chromosomes (balancers) spanning ~75% of the Drosophila genome. Using transheterozyte (balancer/wild-type) embryos, we measured allele-specific changes in topology and gene expression in cis, while minimizing trans effects. Through genome sequencing, we resolved eight large nested inversions, smaller inversions, duplications and thousands of deletions. These extensive rearrangements caused many changes to chromatin topology, disrupting long-range loops, topologically associating domains (TADs) and promoter interactions, yet these are not predictive of changes in expression. Gene expression is generally not altered around inversion breakpoints, indicating that mis-appropriate enhancer-promoter activation is a rare event. Similarly, shuffling or fusing TADs, changing intra-TAD connections and disrupting long-range inter-TAD loops does not alter expression for the majority of genes. Our results suggest that properties other than chromatin topology ensure productive enhancer-promoter interactions.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Comment in
-
A genome disconnect.Nat Genet. 2019 Aug;51(8):1205-1206. doi: 10.1038/s41588-019-0476-x. Nat Genet. 2019. PMID: 31332379 Free PMC article. No abstract available.
-
Toppling TAD tenets.Nat Rev Genet. 2019 Oct;20(10):565. doi: 10.1038/s41576-019-0164-9. Nat Rev Genet. 2019. PMID: 31367009 No abstract available.
References
-
- Spitz F, Furlong EEM. Transcription factors: from enhancer binding to developmental control. Nat Rev Genet. 2012;13:613–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

