Ectopic chondrogenesis of nude mouse induced by nano gene delivery enhanced tissue engineering technology
- PMID: 31308656
- PMCID: PMC6613371
- DOI: 10.2147/IJN.S199306
Ectopic chondrogenesis of nude mouse induced by nano gene delivery enhanced tissue engineering technology
Abstract
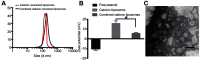
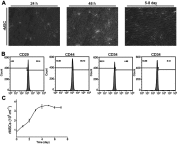
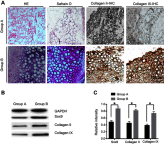
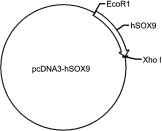
Background: Many techniques and methods have been used clinically to relieve pain from cartilage repair, but the long-term effect is still unsatisfactory. Purpose: The objective of this study was to form an artificial chondroid tissue gene enhanced tissue engineering system to repair cartilage defects via nanosized liposomes. Methods: Cationic nanosized liposomes were prepared and characterized using transmission electron microscope (TEM) and dynamic laser light scattering (DLS). The rat mesenchymal stem cells (rMSCs) were isolated, cultivated, and induced by SRY (Sex-Determining Region Y)-Box 9 (Sox9) via cationic nanosized liposomes. The induced rMSCs were mixed with a thermo-sensitive chitosan hydrogel and subcutaneously injected into the nude mice. Finally, the newly-formed chondroid tissue obtained in the injection parts, and the transparent parts were detected by HE, collagen II, and safranin O. Results: It was found that the presently prepared cationic nanosized liposomes had the diameter of 85.76±3.48 nm and the zeta potential of 15.76±2.1 mV. The isolated rMSCs proliferation was fibroblast-like, with a cultivated confluence of 90% confluence in 5-8 days, and stained positive for CD29 and CD44 while negative for CD34 and CD45. After transfection with cationic nanosized liposomes, we observed changes of cellular morphology and a higher expression of SOX9 compared with control groups, which indicated that rMSCs could differentiate into chondrocyte in vitro. By mixing transfected rMSCs with the thermo-sensitive hydrogel of chitosan in nude mice, chondroid tissue was successfully obtained, demonstrating that rMSCs can differentiate into chondrogenic cells in vivo. Conclusion: This study explored new ways to improve the quality of tissue engineered cartilage, thus accelerating clinical transformation and reducing patient pain.
Keywords: Sox9; chondrogenesis; chondroid; gene enhanced tissue engineering; transfection.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


Similar articles
-
IGF-I induces adipose derived mesenchymal cell chondrogenic differentiation in vitro and enhances chondrogenesis in vivo.In Vitro Cell Dev Biol Anim. 2016 Mar;52(3):356-364. doi: 10.1007/s11626-015-9969-9. Epub 2016 Jan 28. In Vitro Cell Dev Biol Anim. 2016. PMID: 26822434
-
The promotion of cartilage defect repair using adenovirus mediated Sox9 gene transfer of rabbit bone marrow mesenchymal stem cells.Biomaterials. 2011 Jun;32(16):3910-20. doi: 10.1016/j.biomaterials.2011.02.014. Epub 2011 Mar 5. Biomaterials. 2011. PMID: 21377725
-
Differentiation of rodent bone marrow mesenchymal stem cells into intervertebral disc-like cells following coculture with rat disc tissue.Tissue Eng Part A. 2009 Sep;15(9):2581-95. doi: 10.1089/ten.TEA.2008.0458. Tissue Eng Part A. 2009. PMID: 19191570
-
[SOX9 enhanced chondrogenic differentiation potential of human umbilical cord mesenchymal stem cells through cellular aggregation].Zhonghua Yi Xue Za Zhi. 2012 Aug 7;92(29):2050-4. Zhonghua Yi Xue Za Zhi. 2012. PMID: 23253807 Chinese.
-
Role of Liposomes-Based Stem Cell for Multimodal Cancer Therapy.Stem Cell Rev Rep. 2020 Feb;16(1):103-117. doi: 10.1007/s12015-019-09933-z. Stem Cell Rev Rep. 2020. PMID: 31786749 Review.
Cited by
-
Efficient TGF-β1 Delivery to Articular Chondrocytes In Vitro Using Agro-Based Liposomes.Int J Mol Sci. 2022 Mar 5;23(5):2864. doi: 10.3390/ijms23052864. Int J Mol Sci. 2022. PMID: 35270005 Free PMC article.
-
Liposomal Formulations: A Recent Update.Pharmaceutics. 2024 Dec 30;17(1):36. doi: 10.3390/pharmaceutics17010036. Pharmaceutics. 2024. PMID: 39861685 Free PMC article. Review.
References
-
- Babichenko IK. [Cartilage injury of the patella, femoral condyles and knee in trauma to the knee joint]. Ortop Travmatol Protez. 1970;31:34–39. - PubMed
-
- Hofmann FC, Neumann J, Heilmeier U, et al. Conservatively treated knee injury is associated with knee cartilage matrix degeneration measured with MRI-based T2 relaxation times: data from the osteoarthritis initiative. Skeletal Radiol. 2018;47:93–106. doi:10.1007/s00256-017-2759-6 - DOI - PMC - PubMed
-
- Bao CX, Chen HX, Mou XJ, Zhu XK, Zhao Q, Wang XG. GZMB gene silencing confers protection against synovial tissue hyperplasia and articular cartilage tissue injury in rheumatoid arthritis through the MAPK signaling pathway. Biomed Pharmacother. 2018;103:346–354. doi:10.1016/j.biopha.2018.04.023 - DOI - PubMed
-
- Biquet V. [Case of relapsing erythroderma psoriaticum with multiple arthropathies and developing into osteolysis]. Arch Belg Dermatol Syphiligr. 1954;10:58–61. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

