Manganese dioxide nanosheets: from preparation to biomedical applications
- PMID: 31308658
- PMCID: PMC6613456
- DOI: 10.2147/IJN.S207666
Manganese dioxide nanosheets: from preparation to biomedical applications
Abstract
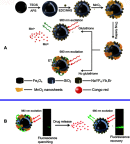
Advancements in nanotechnology and molecular biology have promoted the development of a diverse range of models to intervene in various disorders (from diagnosis to treatment and even theranostics). Manganese dioxide nanosheets (MnO2 NSs), a typical two-dimensional (2D) transition metal oxide of nanomaterial that possesses unique structure and distinct properties have been employed in multiple disciplines in recent decades, especially in the field of biomedicine, including biocatalysis, fluorescence sensing, magnetic resonance imaging and cargo-loading functionality. A brief overview of the different synthetic methodologies for MnO2 NSs and their state-of-the-art biomedical applications is presented below, as well as the challenges and future perspectives of MnO2 NSs.
Keywords: MnO2 nanosheets; biocatalysis; controlled drug delivery; fluorescence sensing; stimuli-activated imaging; synthetic methods.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures









Similar articles
-
Recent advances in functionalized MnO2 nanosheets for biosensing and biomedicine applications.Nanoscale Horiz. 2019 Mar 1;4(2):321-338. doi: 10.1039/c8nh00274f. Epub 2018 Nov 29. Nanoscale Horiz. 2019. PMID: 32254087 Review.
-
Phage capsid protein-directed MnO2 nanosheets with peroxidase-like activity for spectrometric biosensing and evaluation of antioxidant behaviour.Chem Commun (Camb). 2017 May 4;53(37):5216-5219. doi: 10.1039/c7cc02049j. Chem Commun (Camb). 2017. PMID: 28443853
-
Recent Advances in Synthesis and Biomedical Applications of Two-Dimensional Transition Metal Dichalcogenide Nanosheets.Small. 2017 Feb;13(5). doi: 10.1002/smll.201602660. Epub 2016 Dec 16. Small. 2017. PMID: 27982538 Review.
-
MnO2 nanosheets based fluorescent sensing platform with organic dyes as a probe with excellent analytical properties.Analyst. 2015 Jun 21;140(12):4021-9. doi: 10.1039/c5an00581g. Epub 2015 Apr 28. Analyst. 2015. PMID: 25919222
-
The controllable syntheses and electrochemical study of 1-dimensional nanowires, 2-dimensional nanoplatelets, and 3-dimensional nanotowers of MnO2.J Nanosci Nanotechnol. 2007 Jul;7(7):2487-93. doi: 10.1166/jnn.2007.436. J Nanosci Nanotechnol. 2007. PMID: 17663269
Cited by
-
A gold cluster fused manganese dioxide nanocube loaded with dihydroartemisinin for effective cancer treatment via amplified oxidative stress.RSC Adv. 2024 Sep 2;14(38):27703-27711. doi: 10.1039/d4ra03164d. eCollection 2024 Aug 29. RSC Adv. 2024. PMID: 39224649 Free PMC article.
-
MRI Directed Magnevist Effective to Study Toxicity of Gd-Doped Mesoporous Carbon Nanoparticles in Mice Model.Int J Nanomedicine. 2023 Oct 27;18:6119-6136. doi: 10.2147/IJN.S433213. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37915747 Free PMC article.
-
MnO2 Nanoflower Integrated Optoelectronic Biointerfaces for Photostimulation of Neurons.Adv Sci (Weinh). 2023 Sep;10(25):e2301854. doi: 10.1002/advs.202301854. Epub 2023 Jun 29. Adv Sci (Weinh). 2023. PMID: 37386797 Free PMC article.
-
Effects of Mono-Vacancies of Oxygen and Manganese on the Properties of the MnO2/Graphene Heterostructure.Materials (Basel). 2022 Apr 8;15(8):2731. doi: 10.3390/ma15082731. Materials (Basel). 2022. PMID: 35454425 Free PMC article.
-
Two-Dimensional Theranostic Nanomaterials in Cancer Treatment: State of the Art and Perspectives.Cancers (Basel). 2020 Jun 22;12(6):1657. doi: 10.3390/cancers12061657. Cancers (Basel). 2020. PMID: 32580528 Free PMC article. Review.
References
-
- Wang Q, Kalantar-Zadeh K, Kis A, Coleman J, Strano M. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat Nanotechnol. 2012;7(11):699–712. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

