Encapsulation of a nanoporous simvastatin-chitosan composite to enhance osteointegration of hydroxyapatite-coated polyethylene terephthalate ligaments
- PMID: 31308664
- PMCID: PMC6613541
- DOI: 10.2147/IJN.S210687
Encapsulation of a nanoporous simvastatin-chitosan composite to enhance osteointegration of hydroxyapatite-coated polyethylene terephthalate ligaments
Abstract
Purpose: This study was designed to evaluate the in vitro and in vivo biocompatibility and osteointegration of plasma-sprayed hydroxyapatite (HA)-coated polyethylene terephthalate (PET) ligaments encapsulated with a simvastatin (SV)-chitosan (CS) composite.
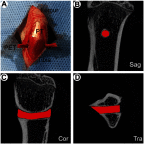
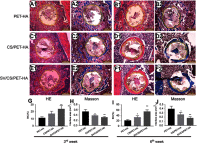
Methods: This study compared the in vitro and in vivo bone responses to three different PET ligaments: SV/CS/PET-HA, CS/PET-HA and PET-HA. A field emission scanning electron microscope was used to characterize the morphology, and the in vitro SV release profile was analyzed. MC3T3 cells were cocultured with SV/CS/PET-HA, CS/PET-HA and PET-HA to test their biocompatibility using CCK-8 tests. Osteogenic differentiation was investigated by the expression of marker genes using qPCR. Osteointegration was performed by implanting the PET ligaments into the proximal tibia bone tunnels of male Sprague-Dawley rats for 3 weeks and 6 weeks. The bone-implant interface was evaluated by micro-computed tomography (micro-CT) and histological analysis.
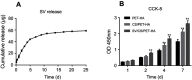
Results: The characteristic nanoporous structures mainly formed on the surface of the plasma-sprayed HA particles in the SV/CS/PET-HA and CS/PET-HA groups. The SV release test showed that the sustained release of simvastatin lasted for 25 days in the SV/CS/PET-HA group. The in vitro studies demonstrated that the SV/CS/PET-HA ligaments induced osteogenic differentiation in the MC3T3 cells, with higher mRNA expression levels of collagen-1, bone morphogenetic protein-2, osteocalcin and alkaline phosphatase than those in the CS/PET-HA and PET-HA ligament groups. The in vivo tests showed that both micro-CT analysis (bone mineral density and bone volume per total volume) and histological analysis (bone implant contact and interface area) revealed significantly higher peri-implant bone formation and less interface area in the SV/CS/PET-HA group than in the other groups.
Conclusion: The SV-CS composite nanoporous structure was associated with the improved biocompatibility and osteogenic differentiation in vitro and enhanced osteointegration process in vivo of plasma-sprayed HA-coated PET ligaments.
Keywords: chitosan; hydroxyapatite; osteointegration; polyethylene terephthalate; simvastatin.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Enhance the biocompatibility and osseointegration of polyethylene terephthalate ligament by plasma spraying with hydroxyapatite in vitro and in vivo.Int J Nanomedicine. 2018 Jun 25;13:3609-3623. doi: 10.2147/IJN.S162466. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29983557 Free PMC article.
-
Enhancement of osseointegration of polyethylene terephthalate artificial ligament by coating of silk fibroin and depositing of hydroxyapatite.Int J Nanomedicine. 2014 Sep 29;9:4569-80. doi: 10.2147/IJN.S69137. eCollection 2014. Int J Nanomedicine. 2014. PMID: 25302023 Free PMC article.
-
Biomineralizaion of hydroxyapatite on polyethylene terephthalate artificial ligaments promotes graft-bone healing after anterior cruciate ligament reconstruction: An in vitro and in vivo study.J Biomater Appl. 2020 Aug;35(2):193-204. doi: 10.1177/0885328220921530. Epub 2020 Apr 26. J Biomater Appl. 2020. PMID: 32338167
-
Local delivery of controlled-release simvastatin to improve the biocompatibility of polyethylene terephthalate artificial ligaments for reconstruction of the anterior cruciate ligament.Int J Nanomedicine. 2016 Jan 27;11:465-78. doi: 10.2147/IJN.S95032. eCollection 2016. Int J Nanomedicine. 2016. PMID: 26869789 Free PMC article.
-
Effects of bioactive strontium-substituted hydroxyapatite on osseointegration of polyethylene terephthalate artificial ligaments.J Mater Chem B. 2021 Sep 7;9(33):6600-6613. doi: 10.1039/d1tb00768h. Epub 2021 Aug 9. J Mater Chem B. 2021. PMID: 34369537
Cited by
-
Preparation and Properties of Antibacterial Polydopamine and Nano-Hydroxyapatite Modified Polyethylene Terephthalate Artificial Ligament.Front Bioeng Biotechnol. 2021 Mar 31;9:630745. doi: 10.3389/fbioe.2021.630745. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33869151 Free PMC article.
-
Establishment of near and non isometric anterior cruciate ligament reconstruction with artificial ligament in a rabbit model.J Orthop Translat. 2021 May 21;29:78-88. doi: 10.1016/j.jot.2021.04.008. eCollection 2021 Jul. J Orthop Translat. 2021. PMID: 34136347 Free PMC article.
-
Engineering Functional Rat Ovarian Spheroids Using Granulosa and Theca Cells.Reprod Sci. 2021 Jun;28(6):1697-1708. doi: 10.1007/s43032-020-00445-7. Epub 2021 Jan 28. Reprod Sci. 2021. PMID: 33511540
-
Effect of chitosan/inorganic nanomaterial scaffolds on bone regeneration and related influencing factors in animal models: A systematic review.Front Bioeng Biotechnol. 2022 Oct 26;10:986212. doi: 10.3389/fbioe.2022.986212. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36394038 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

