Targeted therapy and personalized medicine in gastrointestinal stromal tumors: drug resistance, mechanisms, and treatment strategies
- PMID: 31308690
- PMCID: PMC6612765
- DOI: 10.2147/OTT.S180763
Targeted therapy and personalized medicine in gastrointestinal stromal tumors: drug resistance, mechanisms, and treatment strategies
Abstract
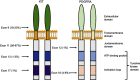
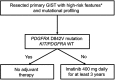
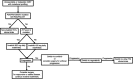
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal tract. Since the discovery that the KIT and PDGFRA receptor tyrosine kinases are the primary oncogenic drivers in the vast majority of GISTs, targeted therapy with tyrosine kinase inhibitors has been the mainstay of treatment for this disease. Using molecular profiling of tumor specimens, researchers also discovered that KIT and PDGFRA mutations are non-random and occur in specific regions of the receptors, and furthermore, that particular genotypes predicted response or resistance to targeted therapy. Imatinib, the first tyrosine kinase inhibitor used to treat GIST, remains the first-line therapy in advanced GIST and the only therapy confirmed through clinical trials in the adjuvant or neoadjuvant setting for resectable disease. Resistance to imatinib is well described and is either primary or secondary. Primary resistance is associated with specific tumor genotypes, so genotyping of individual patient tumors helps guide decision-making into whether to offer imatinib and at what dose. Secondary resistance occurs due to the acquisition of secondary mutations during therapy. Currently, the main strategy to combat imatinib resistance is to switch to another tyrosine kinase inhibitor, because imatinib-resistant GIST is usually still oncogenically addicted to KIT/PDGFRA signaling. Surgery can also be used to combat resistant disease in select settings. Unfortunately, progression-free and overall survival remains dismal for patients who develop imatinib-resistant disease, and further research into alternative strategies is still needed.
Keywords: GIST; imatinib; targeted therapy; tyrosine kinase inhibitor.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Imatinib mesylate: in the treatment of gastrointestinal stromal tumours.Drugs. 2003;63(5):513-22; discussion 523-4. doi: 10.2165/00003495-200363050-00005. Drugs. 2003. PMID: 12600228 Review.
-
[Molecular mechanisms of primary and secondary resistance, molecular-genetic features and characteristics of KIT/PDGFRA non-mutated GISTs].Cesk Patol. 2017 Winter;53(4):167-173. Cesk Patol. 2017. PMID: 29227120 Czech.
-
[The importance of mutational status in prognosis and therapy of GIST].Recenti Prog Med. 2015 Jan;106(1):17-22. doi: 10.1701/1740.18950. Recenti Prog Med. 2015. PMID: 25621775 Italian.
-
Targeting Disease Persistence in Gastrointestinal Stromal Tumors.Stem Cells Transl Med. 2015 Jul;4(7):702-7. doi: 10.5966/sctm.2014-0298. Epub 2015 May 1. Stem Cells Transl Med. 2015. PMID: 25934947 Free PMC article.
-
Tyrosine kinase inhibitors in the treatment of unresectable or metastatic gastrointestinal stromal tumors.Expert Opin Pharmacother. 2014 Oct;15(14):1979-89. doi: 10.1517/14656566.2014.937707. Epub 2014 Jul 3. Expert Opin Pharmacother. 2014. PMID: 24990162 Review.
Cited by
-
Management of Patients With Advanced Gastrointestinal Stromal Tumor: Emphasis on Fourth-Line Treatment With Ripretinib.J Adv Pract Oncol. 2023 May;14(4):317-328. doi: 10.6004/jadpro.2023.14.4.6. Epub 2023 May 1. J Adv Pract Oncol. 2023. PMID: 37313282 Free PMC article.
-
Prediction of recurrence-free survival and adjuvant therapy benefit in patients with gastrointestinal stromal tumors based on radiomics features.Radiol Med. 2022 Oct;127(10):1085-1097. doi: 10.1007/s11547-022-01549-7. Epub 2022 Sep 4. Radiol Med. 2022. PMID: 36057930 Clinical Trial.
-
Identification of New Tumor-Related Gene Mutations in Chinese Gastrointestinal Stromal Tumors.Front Cell Dev Biol. 2021 Nov 3;9:764275. doi: 10.3389/fcell.2021.764275. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34805171 Free PMC article.
-
The Use of Molecular Subtypes for Precision Therapy of Recurrent and Metastatic Gastrointestinal Stromal Tumor.Onco Targets Ther. 2020 Mar 24;13:2433-2447. doi: 10.2147/OTT.S241331. eCollection 2020. Onco Targets Ther. 2020. PMID: 32273716 Free PMC article. Review.
-
Prediction of the mitotic index and preoperative risk stratification of gastrointestinal stromal tumors with CT radiomic features.Radiol Med. 2023 Jun;128(6):644-654. doi: 10.1007/s11547-023-01637-2. Epub 2023 May 6. Radiol Med. 2023. PMID: 37148481 Clinical Trial.
References
-
- Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg. 2006;244:176–184. doi:10.1097/01.sla.0000218080.94145.cf - DOI - PMC - PubMed
-
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. - PubMed
-
- Rubin BP, Singer S, Tsao C, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61:8118–8121. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

