Recombinant human papillomavirus nonavalent vaccine in the prevention of cancers caused by human papillomavirus
- PMID: 31308715
- PMCID: PMC6613616
- DOI: 10.2147/IDR.S178381
Recombinant human papillomavirus nonavalent vaccine in the prevention of cancers caused by human papillomavirus
Abstract
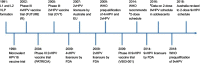
Human papillomavirus (HPV) types 16 and 18 cause 70% of cervical cancer cases globally. The nonavalent HPV vaccine (9vHPV) was licensed in 2014 and protects against the next five most common cancer-causing HPV types (HPV 31/33/45/52/58) after HPV 16/18. Phase III clinical studies have demonstrated high vaccine efficacy (>90%) against cervical, vulvar, and vaginal precancers caused by these additional types, and have shown comparable immunogenicity to the shared genotypes to quadrivalent HPV vaccine (4vHPV). Vaccine efficacy and antibody responses for 9vHPV are found to persist for at least five years while longer-term observational studies are ongoing to monitor long-term vaccine effectiveness. The implementation of 9vHPV has the potential to prevent up to 93% of cervical cancer cases, as well as a significant proportion of other HPV-related anogenital cancers. This review article summarizes the current evidence for 9vHPV in terms of vaccine efficacy against HPV infection and related anogenital precancers, safety, and immunogenicity, as well as discussing the potential impact of this vaccine on the cervical cancer burden globally.
Keywords: efficacy; immunogenicity; nonavalent human papillomavirus vaccine; review; safety.
Conflict of interest statement
FMR and PVL are recipients of National Health and Medical Research Council Fellowships. SMG has received grants through her institution from Merck, GlaxoSmithKline, CSL, and the Commonwealth Department of Health; and has delivered lectures and received speaking fees from MSD for work performed in her personal time. SMG is also a member of Global Advisory Board HPV. The authors report no other conflicts of interest in this work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

