Inconsistency in race and ethnic classification in pharmacogenetics studies and its potential clinical implications
- PMID: 31308725
- PMCID: PMC6612983
- DOI: 10.2147/PGPM.S207449
Inconsistency in race and ethnic classification in pharmacogenetics studies and its potential clinical implications
Abstract
Introduction: Racial and ethnic categories are frequently used in pharmacogenetics literature to stratify patients; however, these categories can be inconsistent across different studies. To address the ongoing debate on the applicability of traditional concepts of race and ethnicity in the context of precision medicine, we aimed to review the application of current racial and ethnic categories in pharmacogenetics and its potential impact on clinical care.
Methods: One hundred and three total pharmacogenetics papers involving the CYP2C9, CYP2C19, and CYP2D6 genes were analyzed for their country of origin, racial, and ethnic categories used, and allele frequency data. Correspondence between the major continental racial categories promulgated by National Institutes of Health (NIH) and those reported by the pharmacogenetics papers was evaluated.
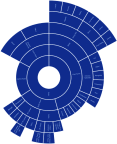
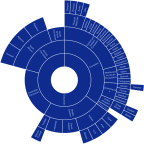
Results: The racial and ethnic categories used in the papers we analyzed were highly heterogeneous. In total, we found 66 different racial and ethnic categories used which fall under the NIH race category "White", 47 different racial and ethnic categories for "Asian", and 62 different categories for "Black". The number of categories used varied widely based on country of origin: Japan used the highest number of different categories for "White" with 17, Malaysia used the highest number for "Asian" with 24, and the US used the highest number for "Black" with 28. Significant variation in allele frequency between different ethnic subgroups was identified within 3 major continental racial categories.
Conclusion: Our analysis showed that racial and ethnic classification is highly inconsistent across different papers as well as between different countries. Evidence-based consensus is necessary for optimal use of self-identified race as well as geographical ancestry in pharmacogenetics. Common taxonomy of geographical ancestry which reflects specifics of particular countries and is accepted by the entire scientific community can facilitate reproducible pharmacogenetic research and clinical implementation of its results.
Keywords: drug responsiveness; ethnic differences; pharmacogenetics; single nucleotide polymorphism.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Results and challenges of Cytochrome P450 2D6 (CYP2D6) testing in an ethnically diverse South Florida population.Mol Genet Genomic Med. 2019 Sep;7(9):e922. doi: 10.1002/mgg3.922. Epub 2019 Aug 7. Mol Genet Genomic Med. 2019. PMID: 31389673 Free PMC article.
-
Race, ethnicity, ancestry, and pharmacogenetics.Mt Sinai J Med. 2010 Mar-Apr;77(2):133-9. doi: 10.1002/msj.20168. Mt Sinai J Med. 2010. PMID: 20309922 Review.
-
Race and ethnicity as variables in Nursing Research, 1952-2000.Nurs Res. 2001 Sep-Oct;50(5):305-13. doi: 10.1097/00006199-200109000-00009. Nurs Res. 2001. PMID: 11570716
-
Pharmacogenetics, race, and ethnicity: social identities and individualized medical care.Ther Drug Monit. 2001 Jun;23(3):232-8. doi: 10.1097/00007691-200106000-00009. Ther Drug Monit. 2001. PMID: 11360031
-
Does race or ethnicity play a role in the origin, pathophysiology, and outcomes of preeclampsia? An expert review of the literature.Am J Obstet Gynecol. 2022 Feb;226(2S):S876-S885. doi: 10.1016/j.ajog.2020.07.038. Epub 2020 Jul 24. Am J Obstet Gynecol. 2022. PMID: 32717255 Review.
Cited by
-
Can pharmacogenetics impact the therapeutic effect of cytarabine and anthracyclines in adult acute myeloid leukaemia patients?: A Serbian experience.J Med Biochem. 2024 Jun 15;43(4):545-555. doi: 10.5937/jomb0-47459. J Med Biochem. 2024. PMID: 39139169 Free PMC article.
-
Demographic diversity of US-based participants in GSK-sponsored interventional clinical trials.Clin Trials. 2023 Apr;20(2):133-144. doi: 10.1177/17407745221149118. Epub 2023 Feb 6. Clin Trials. 2023. PMID: 36744680 Free PMC article.
-
CYP2D6 and CYP2C19 Variant Coverage of Commercial Antidepressant Pharmacogenomic Testing Panels Available in Victoria, Australia.Genes (Basel). 2023 Oct 16;14(10):1945. doi: 10.3390/genes14101945. Genes (Basel). 2023. PMID: 37895294 Free PMC article.
-
Sources of Interindividual Variability.Methods Mol Biol. 2021;2342:481-550. doi: 10.1007/978-1-0716-1554-6_17. Methods Mol Biol. 2021. PMID: 34272705
-
Reductionist methodology and the ambiguity of the categories of race and ethnicity in biomedical research: an exploratory study of recent evidence.Med Health Care Philos. 2023 Mar;26(1):55-68. doi: 10.1007/s11019-022-10122-y. Epub 2022 Nov 9. Med Health Care Philos. 2023. PMID: 36352325 Free PMC article.
References
-
- Center for Drug Evaluation and Research. Table of Pharmacogenomic Biomarkers in Drug Labeling. Maryland: Food and Drug Administration: 2019.
LinkOut - more resources
Full Text Sources

