Identifying risk factors for high-dose methotrexate-induced toxicities in children with acute lymphoblastic leukemia
- PMID: 31308758
- PMCID: PMC6615715
- DOI: 10.2147/CMAR.S207959
Identifying risk factors for high-dose methotrexate-induced toxicities in children with acute lymphoblastic leukemia
Abstract
Background: Whether monitoring of the methotrexate (MTX) concentrations after high-dose MTX (HD-MTX) infusion can predict toxicities is still controversial, especially when HD-MTX therapy is used in the treatment of children with acute lymphoblastic leukemia (ALL), which is different than the previous schedules. The relationship between patient characteristics and severe adverse events (AEs) has yet to be determined.
Objective: To analyze the relationship between the MTX concentration and toxicities and to identify the risk predictors from patient characteristics for severe AEs during HD-MTX therapy in children with ALL.
Methods: We conducted a retrospective study on children with ALL who were treated with 388 HD-MTX infusions. The chi-square test and univariate and logistic regression analyses were used to analyze the relationship between the MTX concentrations and toxicities and to identify predictors for severe AEs.
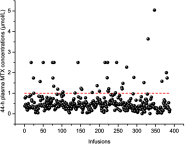
Results: Febrile neutropenia (P=0.000) and vomiting (P=0.034) were more likely to occur if the infusion had an MTX level ≥1 μmol/L at 44 h, but other toxicities had no correlations with MTX concentration. Predictive factors for toxicities were as follows: higher risk stratification and higher values of albumin (ALB) for leucopenia, higher values of white blood cell count (WBC) for anemia, higher values of ALB and creatinine (Cr) for neutropenia, higher risk stratification and higher 44-h MTX concentration for febrile neutropenia, higher values of alanine transferase (ALT) for elevated ALT, higher values of ALT for elevated aspartate transferase (AST), and higher values of total bilirubin (TBil) for vomiting.
Conclusion: Routine monitoring of 44-h MTX concentrations is essential to identify patients at high risk of developing febrile neutropenia and vomiting. This study may provide a reference for clinicians to distinguish patients with a relatively high risk of severe AEs based on certain characteristics before HD-MTX infusion.
Keywords: acute lymphoblastic leukemia; high-dose methotrexate; methotrexate concentration; patient characteristics; risk predictors; toxicities.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Adverse effects with intravenous methotrexate in children with acute lymphoblastic leukemia/lymphoma: a retrospective study.Indian J Hematol Blood Transfus. 2020 Jul;36(3):498-504. doi: 10.1007/s12288-019-01245-z. Epub 2020 Jan 2. Indian J Hematol Blood Transfus. 2020. PMID: 32647424 Free PMC article.
-
Involvement of the ABCB1 C3435T Variant but Not the MTHFR C677T or MTHFR A1298C Variant in High-Dose Methotrexate-Induced Toxicity in Pediatric Acute Lymphoblastic Leukemia Patients in China.Int J Gen Med. 2024 Mar 27;17:1221-1231. doi: 10.2147/IJGM.S453394. eCollection 2024. Int J Gen Med. 2024. PMID: 38559593 Free PMC article.
-
Solitary serum methotrexate level 36 hours post high-dose methotrexate: A safe, efficacious, and cost-effective strategy to monitor methotrexate toxicities in childhood leukemia in resource-limited centers.Pediatr Blood Cancer. 2020 Jul;67(7):e28387. doi: 10.1002/pbc.28387. Epub 2020 May 13. Pediatr Blood Cancer. 2020. PMID: 32400952
-
Association between high-dose methotrexate-induced toxicity and polymorphisms within methotrexate pathway genes in acute lymphoblastic leukemia.Front Pharmacol. 2022 Nov 30;13:1003812. doi: 10.3389/fphar.2022.1003812. eCollection 2022. Front Pharmacol. 2022. PMID: 36532750 Free PMC article. Review.
-
Association of microRNA Polymorphisms with Toxicities Induced by Methotrexate in Children with Acute Lymphoblastic Leukemia.Hematol Rep. 2023 Nov 20;15(4):634-650. doi: 10.3390/hematolrep15040065. Hematol Rep. 2023. PMID: 37987321 Free PMC article. Review.
Cited by
-
Integration of genomics, clinical characteristics and baseline biological profiles to predict the risk of liver injury induced by high-dose methotrexate.Front Pharmacol. 2024 Nov 28;15:1423214. doi: 10.3389/fphar.2024.1423214. eCollection 2024. Front Pharmacol. 2024. PMID: 39669197 Free PMC article.
-
Melissa Officinalis L. aqueous extract pretreatment decreases methotrexate-induced hepatotoxicity at lower dose and increases 99mTc-phytate liver uptake, as a probe of liver toxicity assessment, in rats.Ann Nucl Med. 2023 Mar;37(3):166-175. doi: 10.1007/s12149-022-01813-w. Epub 2022 Dec 5. Ann Nucl Med. 2023. PMID: 36469234
-
Ethnic-specific predictors of neurotoxicity among patients with pediatric acute lymphoblastic leukemia after high-dose methotrexate.Cancer. 2023 Apr 15;129(8):1287-1294. doi: 10.1002/cncr.34646. Epub 2023 Jan 24. Cancer. 2023. PMID: 36692972 Free PMC article.
-
Population pharmacokinetic model of high-dose methotrexate in Chinese patients with intracranial germ cell tumors.Front Pharmacol. 2025 May 2;16:1548203. doi: 10.3389/fphar.2025.1548203. eCollection 2025. Front Pharmacol. 2025. PMID: 40385482 Free PMC article.
-
Nomogram predicting leukopenia in osteosarcoma after high-dose methotrexate chemotherapy.Aging (Albany NY). 2022 May 31;14(12):5023-5033. doi: 10.18632/aging.203978. Epub 2022 May 31. Aging (Albany NY). 2022. PMID: 35640086 Free PMC article.
References
-
- Abromowitch M, Ochs J, Pui CH, et al. High-dose methotrexate improves clinical outcome in children with acute lymphoblastic leukemia: st. Jude total therapy study X. Med Pediatr Oncol. 1988;16(5):297–303. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous