Efficacy, safety, and quality-of-life of treatments for acute relapses of multiple sclerosis: results from a literature review of randomized controlled trials
- PMID: 31308790
- PMCID: PMC6613013
- DOI: 10.2147/DNND.S208815
Efficacy, safety, and quality-of-life of treatments for acute relapses of multiple sclerosis: results from a literature review of randomized controlled trials
Abstract
Background: Intravenous methylprednisolone (IVMP), repository corticotropin injection (RCI), plasmapheresis (PMP), and intravenous immunoglobulin (IVIG) are used in the treatment of acute multiple sclerosis (MS) relapse. A systematic literature review (SLR) of randomized controlled trials (RCTs) was conducted to examine the highest quality evidence available for these therapies.
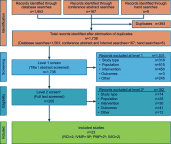
Methods: English-language articles were searched in MEDLINE, Embase, and Cochrane Library through May 2016 per Preferred Reporting Items for Systematic Reviews and Meta-Analyses standards. MS conferences, SLRs, and bibliographies of included studies were also searched. Eligible studies included adults treated with ≥1 aforementioned therapy.
Results: Twenty-three RCTs were identified: 22 on efficacy, 11 on safety, and 3 on QOL (ie 18 IVMP, 2 RCI, 2 PMP, and 2 IVIG). IVMP and RCI improved relapse-related disability; however, IVIG and PMP showed inconsistent efficacy. QOL data were only ascertained for IVMP.
Conclusions: RCTs indicate IVMP and RCI are efficacious and well tolerated treatments for MS relapse. Overall, many RCTs were dated, with sample sizes of fewer than 30 patients and no definitions for relapse nor clinically significant change. Contemporary evidence generation for all relapse treatments of interest, across efficacy, safety, and QOL outcomes, is still needed.
Keywords: efficacy; multiple sclerosis relapse; safety; systematic literature review.
Conflict of interest statement
Jessica Costello, Annete Njue, Matthew Lyall, and Anne Heyes were employees of RTI Health Solutions at the time this SLR was developed and provided consulting services to Mallinckrodt Pharmaceuticals. Nancy Mahler is an employee of Mallinckrodt Pharmaceuticals. Tara Nazareth and Michael Philbin were employees of Mallinckrodt Pharmaceuticals at the time this work was done. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Cost-Effectiveness of Repository Corticotropin Injection for the Treatment of Acute Exacerbations in Multiple Sclerosis.Clinicoecon Outcomes Res. 2021 Oct 11;13:883-892. doi: 10.2147/CEOR.S330118. eCollection 2021. Clinicoecon Outcomes Res. 2021. PMID: 34675568 Free PMC article.
-
Treatment Effectiveness for Resolution of Multiple Sclerosis Relapse in a US Health Plan Population.Neurol Ther. 2019 Dec;8(2):383-395. doi: 10.1007/s40120-019-00156-5. Epub 2019 Sep 28. Neurol Ther. 2019. PMID: 31564036 Free PMC article.
-
Oral versus intravenous methylprednisolone for the treatment of multiple sclerosis relapses: A meta-analysis of randomized controlled trials.PLoS One. 2017 Nov 27;12(11):e0188644. doi: 10.1371/journal.pone.0188644. eCollection 2017. PLoS One. 2017. PMID: 29176905 Free PMC article.
-
Indirect-comparison meta-analysis of treatment options for patients with refractory Kawasaki disease.BMC Pediatr. 2019 May 17;19(1):158. doi: 10.1186/s12887-019-1504-9. BMC Pediatr. 2019. PMID: 31101091 Free PMC article. Review.
-
Re-examining the effects of high-dose intravenous methylprednisolone for secondary progressive multiple sclerosis.Neurodegener Dis Manag. 2021 Apr;11(2):177-185. doi: 10.2217/nmt-2020-0051. Epub 2021 Mar 11. Neurodegener Dis Manag. 2021. PMID: 33703936
Cited by
-
Effect of Health Care Providers' Focused Discussion and Proactive Education About Relapse Management on Patient Reporting of Multiple Sclerosis Relapse.Int J MS Care. 2021 Jul-Aug;23(4):151-156. doi: 10.7224/1537-2073.2020-018. Epub 2021 Feb 8. Int J MS Care. 2021. PMID: 34483753 Free PMC article.
-
Cost-Effectiveness of Repository Corticotropin Injection for the Treatment of Acute Exacerbations in Multiple Sclerosis.Clinicoecon Outcomes Res. 2021 Oct 11;13:883-892. doi: 10.2147/CEOR.S330118. eCollection 2021. Clinicoecon Outcomes Res. 2021. PMID: 34675568 Free PMC article.
-
An interactive web-based programme on relapse management for people with multiple sclerosis (POWER@MS2) - development, feasibility, and pilot testing of a complex intervention.Front Neurol. 2022 Sep 23;13:914814. doi: 10.3389/fneur.2022.914814. eCollection 2022. Front Neurol. 2022. PMID: 36212638 Free PMC article.
-
Consensus Statement On Immune Modulation in Multiple Sclerosis and Related Disorders During the COVID-19 Pandemic: Expert Group on Behalf of the Indian Academy of Neurology.Ann Indian Acad Neurol. 2020 Apr;23(Suppl 1):S5-S14. doi: 10.4103/0972-2327.282442. Epub 2020 Apr 13. Ann Indian Acad Neurol. 2020. PMID: 32419748 Free PMC article.
-
Cost per response analysis of repository corticotropin injection versus other alternative treatments for acute exacerbations of multiple sclerosis.Drugs Context. 2020 Dec 16;9:2020-9-4. doi: 10.7573/dic.2020-9-4. eCollection 2020. Drugs Context. 2020. PMID: 33408750 Free PMC article.
References
-
- Bernard JT. Treating Acute Relapses in MS. CME released MedScape; March 17, 2015. Available from: https://www.medscape.org/viewarticle/839227. Accessed June 14, 2019.
-
- Food and Drug Administration. SOLU-MEDROL® (Methylprednisolone Sodium Succinate for Injection, USP). FDA; 2011. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/011856s103s104.... Accessed June 14, 2019.
LinkOut - more resources
Full Text Sources

