Memantine Improves Cognitive Function and Alters Hippocampal and Cortical Proteome in Triple Transgenic Mouse Model of Alzheimer's Disease
- PMID: 31308798
- PMCID: PMC6614075
- DOI: 10.5607/en.2019.28.3.390
Memantine Improves Cognitive Function and Alters Hippocampal and Cortical Proteome in Triple Transgenic Mouse Model of Alzheimer's Disease
Abstract
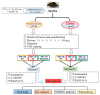
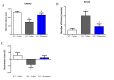
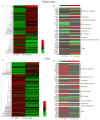
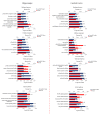
Memantine is a non-competitive N-methyl-D-aspartate receptor (NMDAR) antagonist clinically approved for moderate-to-severe Alzheimer's disease (AD) to improve cognitive functions. There is no report about the proteomic alterations induced by memantine in AD mouse model yet. In this study, we investigated the protein profiles in the hippocampus and the cerebral cortex of AD-related transgenic mouse model (3×Tg-AD) treated with memantine. Mice (8-month) were treated with memantine (5 mg/kg/bid) for 4 months followed by behavioral and molecular evaluation. Using step-down passive avoidance (SDA) test, novel object recognition (NOR) test and Morris water maze (MWM) test, it was observed that memantine significantly improved learning and memory retention in 3xTg-AD mice. By using quantitative proteomic analysis, 3301 and 3140 proteins in the hippocampus and the cerebral cortex respectively were identified to be associated with AD abnormalities. In the hippocampus, memantine significantly altered the expression levels of 233 proteins, among which PCNT, ATAXIN2, TNIK, and NOL3 were up-regulated, and FLNA, MARK 2 and BRAF were down-regulated. In the cerebral cortex, memantine significantly altered the expression levels of 342 proteins, among which PCNT, PMPCB, CRK, and MBP were up-regulated, and DNM2, BRAF, TAGLN 2 and FRY1 were down-regulated. Further analysis with bioinformatics showed that memantine modulated biological pathways associated with cytoskeleton and ErbB signaling in the hippocampus, and modulated biological pathways associated with axon guidance, ribosome, cytoskeleton, calcium and MAPK signaling in the cerebral cortex. Our data indicate that memantine induces higher levels of proteomic alterations in the cerebral cortex than in the hippocampus, suggesting memantine affects various brain regions in different manners. Our study provides a novel view on the complexity of protein responses induced by memantine in the brain of AD.
Keywords: Alzheimer's disease (AD); MARK2; Memantine; Proteomic.
Figures



Similar articles
-
Hippocampal Proteomic Alteration in Triple Transgenic Mouse Model of Alzheimer's Disease and Implication of PINK 1 Regulation in Donepezil Treatment.J Proteome Res. 2019 Apr 5;18(4):1542-1552. doi: 10.1021/acs.jproteome.8b00818. Epub 2018 Dec 7. J Proteome Res. 2019. PMID: 30484658
-
Tetramethylpyrazine Improves Cognitive Impairment and Modifies the Hippocampal Proteome in Two Mouse Models of Alzheimer's Disease.Front Cell Dev Biol. 2021 Mar 15;9:632843. doi: 10.3389/fcell.2021.632843. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33791294 Free PMC article.
-
Identification of the key molecules involved in chronic copper exposure-aggravated memory impairment in transgenic mice of Alzheimer's disease using proteomic analysis.J Alzheimers Dis. 2015;44(2):455-69. doi: 10.3233/JAD-141776. J Alzheimers Dis. 2015. PMID: 25352456
-
Characterization of cognitive deficits in a transgenic mouse model of Alzheimer's disease and effects of donepezil and memantine.Eur J Pharmacol. 2013 Mar 5;703(1-3):53-61. doi: 10.1016/j.ejphar.2012.12.023. Epub 2012 Dec 28. Eur J Pharmacol. 2013. PMID: 23276665
-
Assessment of Cognitive Phenotyping in Inbred, Genetically Modified Mice, and Transgenic Mouse Models of Alzheimer's Disease.Exp Neurobiol. 2019 Apr;28(2):146-157. doi: 10.5607/en.2019.28.2.146. Epub 2019 Apr 8. Exp Neurobiol. 2019. PMID: 31138986 Free PMC article. Review.
Cited by
-
Effects of long-term low dose saxitoxin exposure on nerve damage in mice.Aging (Albany NY). 2021 Jul 1;13(13):17211-17226. doi: 10.18632/aging.203199. Epub 2021 Jul 1. Aging (Albany NY). 2021. PMID: 34197336 Free PMC article.
-
Basic information about memantine and its treatment of Alzheimer's disease and other clinical applications.Ibrain. 2023 Jun 6;9(3):340-348. doi: 10.1002/ibra.12098. eCollection 2023 Fall. Ibrain. 2023. PMID: 37786758 Free PMC article. Review.
-
Pharmacogenomics of Cognitive Dysfunction and Neuropsychiatric Disorders in Dementia.Int J Mol Sci. 2020 Apr 26;21(9):3059. doi: 10.3390/ijms21093059. Int J Mol Sci. 2020. PMID: 32357528 Free PMC article. Review.
-
Intranasal Administration of Apelin-13 Ameliorates Cognitive Deficit in Streptozotocin-Induced Alzheimer's Disease Model via Enhancement of Nrf2-HO1 Pathways.Brain Sci. 2024 May 11;14(5):488. doi: 10.3390/brainsci14050488. Brain Sci. 2024. PMID: 38790466 Free PMC article.
-
Actin-binding protein filamin-A drives tau aggregation and contributes to progressive supranuclear palsy pathology.Sci Adv. 2022 May 27;8(21):eabm5029. doi: 10.1126/sciadv.abm5029. Epub 2022 May 25. Sci Adv. 2022. PMID: 35613261 Free PMC article.
References
-
- Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ Memantine Study Group. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348:1333–1341. - PubMed
-
- Scholtzova H, Wadghiri YZ, Douadi M, Sigurdsson EM, Li YS, Quartermain D, Banerjee P, Wisniewski T. Memantine leads to behavioral improvement and amyloid reduction in Alzheimer's-disease-model transgenic mice shown as by micromagnetic resonance imaging. J Neurosci Res. 2008;86:2784–2791. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

