Differentiation and identification of ginsenoside structural isomers by two-dimensional mass spectrometry combined with statistical analysis
- PMID: 31308808
- PMCID: PMC6606828
- DOI: 10.1016/j.jgr.2017.11.002
Differentiation and identification of ginsenoside structural isomers by two-dimensional mass spectrometry combined with statistical analysis
Abstract
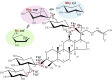
Background: In the current phytochemical research on ginseng, the differentiation and structural identification of ginsenosides isomers remain challenging. In this paper, a two-dimensional mass spectrometry (2D-MS) method was developed and combined with statistical analysis for the direct differentiation, identification, and relative quantification of protopanaxadiol (PPD)-type ginsenoside isomers.
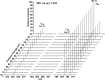
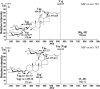
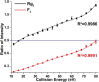
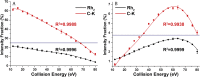
Methods: Collision-induced dissociation was performed at successive collision energy values to produce distinct profiles of the intensity fraction (IF) and ratio of intensity (RI) of the fragment ions. To amplify the differences in tandem mass spectra between isomers, IF and RI were plotted against collision energy. The resulting data distributions were then used to obtain the parameters of the fitted curves, which were used to evaluate the statistical significance of the differences between these distributions via the unpaired t test.
Results: A triplet and two pairs of PPD-type ginsenoside isomers were differentiated and identified by their distinct IF and RI distributions. In addition, the fragmentation preference of PPD-type ginsenosides was determined on the basis of the activation energy. The developed 2D-MS method was also extended to quantitatively determine the molar composition of ginsenoside isomers in mixtures of biotransformation products.
Conclusion: In comparison with conventional mass spectrometry methods, 2D-MS provides more direct insights into the subtle structural differences between isomers and can be used as an alternative approach for the differentiation of isomeric ginsenosides and natural products.
Keywords: Differentiation; Ginsenoside isomers; Relative quantification; Two-dimensional mass spectrometry.
Figures



Similar articles
-
Silver (Ι)-assisted enantiomeric analysis of ginsenosides using electrospray ionization tandem mass spectrometry.J Mass Spectrom. 2012 Oct;47(10):1313-21. doi: 10.1002/jms.3085. J Mass Spectrom. 2012. PMID: 23019162
-
Characterization of ginsenoside structural isomers from mixtures using in situ methylation with direct analysis in real-time ionization tandem mass spectrometry.Anal Bioanal Chem. 2023 Feb;415(5):887-897. doi: 10.1007/s00216-022-04482-w. Epub 2022 Dec 26. Anal Bioanal Chem. 2023. PMID: 36571591
-
Biotransformation of Ginsenoside Rb1 to Ginsenoside Rd and 7 Rare Ginsenosides Using Irpex lacteus with HPLC-HRMS/MS Identification.ACS Omega. 2024 May 16;9(21):22744-22753. doi: 10.1021/acsomega.4c00837. eCollection 2024 May 28. ACS Omega. 2024. PMID: 38826525 Free PMC article.
-
Advances in Saponin Diversity of Panax ginseng.Molecules. 2020 Jul 29;25(15):3452. doi: 10.3390/molecules25153452. Molecules. 2020. PMID: 32751233 Free PMC article. Review.
-
Collisional activation of peptide ions in FT-ICR mass spectrometry.Mass Spectrom Rev. 2003 May-Jun;22(3):158-81. doi: 10.1002/mas.10041. Mass Spectrom Rev. 2003. PMID: 12838543 Review.
Cited by
-
Effect of red ginseng NaturalGEL on skin aging.J Ginseng Res. 2020 Jan;44(1):115-122. doi: 10.1016/j.jgr.2018.09.006. Epub 2018 Oct 6. J Ginseng Res. 2020. PMID: 32148394 Free PMC article.
-
Ginsenoside M1 Induces Apoptosis and Inhibits the Migration of Human Oral Cancer Cells.Int J Mol Sci. 2020 Dec 19;21(24):9704. doi: 10.3390/ijms21249704. Int J Mol Sci. 2020. PMID: 33352689 Free PMC article.
-
Anticancer Activities of Ginsenosides, the Main Active Components of Ginseng.Evid Based Complement Alternat Med. 2021 Feb 3;2021:8858006. doi: 10.1155/2021/8858006. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 33623532 Free PMC article. Review.
-
Rare ginsenosides: A unique perspective of ginseng research.J Adv Res. 2024 Dec;66:303-328. doi: 10.1016/j.jare.2024.01.003. Epub 2024 Jan 7. J Adv Res. 2024. PMID: 38195040 Free PMC article. Review.
-
Triple three-dimensional MS/MS spectrum facilitates quantitative ginsenosides-targeted sub-metabolome characterization in notoginseng.Acta Pharm Sin B. 2024 Sep;14(9):4045-4058. doi: 10.1016/j.apsb.2024.04.029. Epub 2024 May 3. Acta Pharm Sin B. 2024. PMID: 39309494 Free PMC article.
References
-
- State Pharmacopoeia Committee . China Medical Science and Technology Press; Beijing: 2010. Pharmacopoeia of the People’s Republic of China.
-
- Liu Z.Q. Chemical insights into ginseng as a resource for natural antioxidants. Chem Rev. 2012;112:3329–3355. - PubMed
-
- Ganesan P., Ko H.M., Kim I.S., Choi D.K. Recent trends of nano bioactive compounds from ginseng for its possible preventive role in chronic disease models. RSC Adv. 2015;5:98634–98642.
-
- Wong A.S.T., Che C.M., Leung K.W. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat Prod Rep. 2015;32:256–272. - PubMed
-
- Li K.K., Gong X.J. A review on the medicinal potential of Panax ginseng saponins in diabetes mellitus. RSC Adv. 2015;5:47353–47366.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

