Expression of Toll-Like Receptors (TLR2 and TLR4) in the Eyes of Mice with Disseminated Acanthamoebiasis
- PMID: 31309100
- PMCID: PMC6594330
- DOI: 10.1155/2019/1401894
Expression of Toll-Like Receptors (TLR2 and TLR4) in the Eyes of Mice with Disseminated Acanthamoebiasis
Abstract
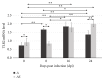
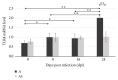
Toll-like receptors (TLRs) play a key role in the innate immune response to numerous pathogens, including Acanthamoeba spp. The aim of this study was to determine the expression of TLR2 and TLR4 in the eyes of mice following intranasal infection with Acanthamoeba spp. in relation to the host's immunological status. Amoebae used in this study were isolated from the bronchial aspirate of a patient with acute myeloid leukemia (AML) and atypical symptoms of pneumonia. We found statistically significant differences in the expression of TLR2 and TLR4 in the eye of immunocompetent mice at 8, 16, and 24 days after Acanthamoeba spp. infection (dpi) compared to control group. Immunosuppressed mice showed significant differences in the expression of TLR2 at 16 and 24 dpi compared to uninfected animals. Our results indicate that TLR2 and TLR4 are upregulated in the eyes of mice in response to Acanthamoeba spp. We suggest that it is possible for trophozoites to migrate through the optic nerve from the brain to the eyes. The course of disseminated acanthamoebiasis may be influenced by the host's immunological status, and the observed changes in expression of TLR2 and TLR4 in the host's organs may indicate the role of these receptors in the pathomechanism of acanthamoebiasis.
Figures


Similar articles
-
The expression of TLR2 and TLR4 in the kidneys and heart of mice infected with Acanthamoeba spp.Parasit Vectors. 2020 Sep 21;13(1):480. doi: 10.1186/s13071-020-04351-4. Parasit Vectors. 2020. PMID: 32958053 Free PMC article.
-
Toll-like receptors in the brain of mice following infection with Acanthamoeba spp.Parasitol Res. 2016 Nov;115(11):4335-4344. doi: 10.1007/s00436-016-5217-9. Epub 2016 Aug 11. Parasitol Res. 2016. PMID: 27511368 Free PMC article.
-
Acanthamoeba infection in lungs of mice expressed by toll-like receptors (TLR2 and TLR4).Exp Parasitol. 2016 Jun;165:30-4. doi: 10.1016/j.exppara.2016.02.012. Epub 2016 Mar 3. Exp Parasitol. 2016. PMID: 26940205
-
Changes in the immune system in experimental acanthamoebiasis in immunocompetent and immunosuppressed hosts.Parasit Vectors. 2018 Sep 20;11(1):517. doi: 10.1186/s13071-018-3108-x. Parasit Vectors. 2018. PMID: 30236160 Free PMC article.
-
Immunopathogenicity of Acanthamoeba spp. in the Brain and Lungs.Int J Mol Sci. 2021 Jan 27;22(3):1261. doi: 10.3390/ijms22031261. Int J Mol Sci. 2021. PMID: 33514026 Free PMC article. Review.
Cited by
-
Foundational concepts in the biology of bacterial keratitis.Exp Eye Res. 2021 Aug;209:108647. doi: 10.1016/j.exer.2021.108647. Epub 2021 Jun 5. Exp Eye Res. 2021. PMID: 34097906 Free PMC article. Review.
-
Microbial Signatures in The Rodent Eyes With Retinal Dysfunction and Diabetic Retinopathy.Invest Ophthalmol Vis Sci. 2022 Jan 3;63(1):5. doi: 10.1167/iovs.63.1.5. Invest Ophthalmol Vis Sci. 2022. PMID: 34985498 Free PMC article.
-
Antioxidant defense in the eyes of immunocompetent and immunosuppressed mice infected with Acanthamoeba spp.Parasit Vectors. 2020 Mar 7;13(1):123. doi: 10.1186/s13071-020-3979-5. Parasit Vectors. 2020. PMID: 32143731 Free PMC article.
-
The Immunological Changes in the Skin of BALC/c Mice with Disseminated Acanthamoebiasis.Pathogens. 2023 Apr 22;12(5):631. doi: 10.3390/pathogens12050631. Pathogens. 2023. PMID: 37242301 Free PMC article.
-
Concentrations of PGE2 and TXB2 in the Eyes of Mice with Disseminated Acanthamoebiasis.Pathogens. 2022 Apr 4;11(4):438. doi: 10.3390/pathogens11040438. Pathogens. 2022. PMID: 35456113 Free PMC article.
References
-
- Mishra B. B., Gundra U. M., Teale J. M. Toll-like receptors in CNS parasitic infections. Current Topics in Microbiology and Immunology. 2009;336:83–104. - PubMed
-
- De Jonckheere J. F. Ecology of Acanthamoeba. Reviews of Infectious Diseases. 1991;13:S385–S387. - PubMed
-
- Cerva L. Acanthamoeba culbertsoni and Naegleria fowleri: occurrence of antibodies in man. Journal of Hygiene, Epidemiology, Microbiology, and Immunology. 1989;33(1):99–103. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

