Enhancement of Adeno-Associated Virus-Mediated Gene Therapy Using Hydroxychloroquine in Murine and Human Tissues
- PMID: 31309129
- PMCID: PMC6606965
- DOI: 10.1016/j.omtm.2019.05.012
Enhancement of Adeno-Associated Virus-Mediated Gene Therapy Using Hydroxychloroquine in Murine and Human Tissues
Erratum in
-
Erratum: Enhancement of Adeno-Associated Virus-Mediated Gene Therapy Using Hydroxychloroquine in Murine and Human Tissues.Mol Ther Methods Clin Dev. 2023 Feb 13;28:300. doi: 10.1016/j.omtm.2023.01.001. eCollection 2023 Mar 9. Mol Ther Methods Clin Dev. 2023. PMID: 36843676 Free PMC article.
Abstract
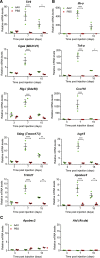
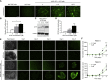
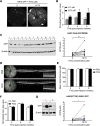
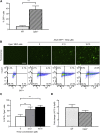
The therapeutic effects of gene therapy using adeno-associated virus (AAV) vectors are dependent on the efficacy of viral transduction. Currently, we have reached the safe limits of AAV vector dose, beyond which damaging inflammatory responses are seen. To improve the efficacy of AAV transduction, we treated mouse embryonic fibroblasts, primate retinal pigment epithelial cells, and human retinal explants with hydroxychloroquine (HCQ) 1 h prior to transduction with an AAV2 vector encoding GFP driven by a ubiquitous CAG promoter. This led to a consistent increase in GFP expression, up to 3-fold, compared with vector alone. Comparing subretinal injections of AAV2.CAG.GFP vector alone versus co-injection with 18.75 μM HCQ in paired eyes in mice, mean GFP expression was 4.6-fold higher in retinae co-treated with HCQ without retinal toxicity. A comparative 5.9-fold effect was seen with an AAV8(Y733F).GRK1.GFP vector containing the photoreceptor-specific rhodopsin kinase promoter. While the mechanism of action remains to be fully elucidated, our data suggest that a single pulse of adjunctive HCQ could safely improve AAV transduction in vivo, thus providing a novel strategy for enhancing the clinical effects of gene therapy.
Keywords: AAV; APOBEC; TLR9; Toll-like receptor 9; adeno-associated virus; cGAS; chloroquine; gene therapy; hydroxychloroquine; innate immunity.
Figures

References
-
- Bainbridge J.W., Smith A.J., Barker S.S., Robbie S., Henderson R., Balaggan K., Viswanathan A., Holder G.E., Stockman A., Tyler N. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

