Nerve injury drives a heightened state of vigilance and neuropathic sensitization in Drosophila
- PMID: 31309148
- PMCID: PMC6620091
- DOI: 10.1126/sciadv.aaw4099
Nerve injury drives a heightened state of vigilance and neuropathic sensitization in Drosophila
Abstract
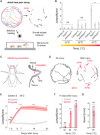
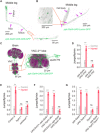
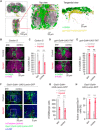
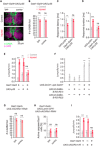
Injury can lead to devastating and often untreatable chronic pain. While acute pain perception (nociception) evolved more than 500 million years ago, virtually nothing is known about the molecular origin of chronic pain. Here we provide the first evidence that nerve injury leads to chronic neuropathic sensitization in insects. Mechanistically, peripheral nerve injury triggers a loss of central inhibition that drives escape circuit plasticity and neuropathic allodynia. At the molecular level, excitotoxic signaling within GABAergic (γ-aminobutyric acid) neurons required the acetylcholine receptor nAChRα1 and led to caspase-dependent death of GABAergic neurons. Conversely, disruption of GABA signaling was sufficient to trigger allodynia without injury. Last, we identified the conserved transcription factor twist as a critical downstream regulator driving GABAergic cell death and neuropathic allodynia. Together, we define how injury leads to allodynia in insects, and describe a primordial precursor to neuropathic pain may have been advantageous, protecting animals after serious injury.
Figures

Similar articles
-
TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord.Neuron. 2012 May 24;74(4):640-7. doi: 10.1016/j.neuron.2012.02.039. Neuron. 2012. PMID: 22632722
-
The etiological contribution of GABAergic plasticity to the pathogenesis of neuropathic pain.Mol Pain. 2019 Jan-Dec;15:1744806919847366. doi: 10.1177/1744806919847366. Mol Pain. 2019. PMID: 30977423 Free PMC article. Review.
-
Neuropathic Pain: Sensory Nerve Injury or Motor Nerve Injury?Adv Exp Med Biol. 2016;904:59-75. doi: 10.1007/978-94-017-7537-3_5. Adv Exp Med Biol. 2016. PMID: 26900063 Review.
-
Nox2-dependent signaling between macrophages and sensory neurons contributes to neuropathic pain hypersensitivity.Pain. 2014 Oct;155(10):2161-70. doi: 10.1016/j.pain.2014.08.013. Epub 2014 Aug 17. Pain. 2014. PMID: 25139590
-
The role of keratinocyte-derived chemokine (KC) on hyperalgesia caused by peripheral nerve injury in mice.Neuropharmacology. 2014 Apr;79:17-27. doi: 10.1016/j.neuropharm.2013.10.026. Epub 2013 Nov 1. Neuropharmacology. 2014. PMID: 24184386
Cited by
-
Injury alters motivational trade-offs in calves during the healing period.Sci Rep. 2021 Mar 25;11(1):6888. doi: 10.1038/s41598-021-86313-z. Sci Rep. 2021. PMID: 33767288 Free PMC article.
-
Edible Insects as Food-Insect Welfare and Ethical Aspects from a Consumer Perspective.Insects. 2022 Jan 25;13(2):121. doi: 10.3390/insects13020121. Insects. 2022. PMID: 35206696 Free PMC article. Review.
-
Human Pluripotent Stem Cells-Based Therapies for Neurodegenerative Diseases: Current Status and Challenges.Cells. 2020 Nov 20;9(11):2517. doi: 10.3390/cells9112517. Cells. 2020. PMID: 33233861 Free PMC article. Review.
-
Drosophila as a Model to Study the Mechanism of Nociception.Front Physiol. 2022 Mar 28;13:854124. doi: 10.3389/fphys.2022.854124. eCollection 2022. Front Physiol. 2022. PMID: 35418874 Free PMC article. Review.
-
Peripheral straightjacket (α2δ Ca2+ channel subunit) expression is required for neuropathic sensitization in Drosophila.Philos Trans R Soc Lond B Biol Sci. 2019 Nov 11;374(1785):20190287. doi: 10.1098/rstb.2019.0287. Epub 2019 Sep 23. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31544607 Free PMC article.
References
-
- Turk D. C., Wilson H. D., Cahana A., Treatment of chronic non-cancer pain. Lancet 377, 2226–2235 (2011). - PubMed
-
- Institute of Medicine, Board on Health Sciences Policy, Committee on Advancing Pain Research, Care, and Education in Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research (National Academies Press, 2011). - PubMed
-
- Finnerup N. B., Haroutounian S., Kamerman P., Baron R., Bennett D. L. H., Bouhassira D., Cruccu G., Freeman R., Hansson P., Nurmikko T., Raja S. N., Rice A. S., Serra J., Smith B. H., Treede R. D., Jensen T. S., Neuropathic pain: An updated grading system for research and clinical practice. Pain 157, 1599–1606 (2016). - PMC - PubMed
-
- Grosser T., Woolf C. J., FitzGerald G. A., Time for nonaddictive relief of pain. Science 355, 1026–1027 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

