Novel TRIM32 mutation in sarcotubular myopathy
- PMID: 31309175
- PMCID: PMC6598407
Novel TRIM32 mutation in sarcotubular myopathy
Abstract
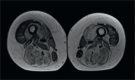
Tripartite motif-containing protein 32 (TRIM32) is a member of the TRIM ubiquitin E3 ligases which ubiquitinates different substrates in muscle including sarcomeric proteins. Mutations in TRIM32 are associated with Limb-Girdle Muscular Dystrophy 2H. In a 66 old woman with disto-proximal myopathy, we identified a novel homozygous mutation of TRIM32 gene c.1781G > A (p. Ser594Asn) localised in the c-terminus NHL domain. Mutations of this domain have been also associated to Sarcotubular Myopathy (STM), a form of distal myopathy with peculiar features in muscle biopsy, now considered in the spectrum of LGMD2H. Muscle biopsy revealed severe abnormalities of the myofibrillar network with core like areas, lobulated fibres, whorled fibres and multiple vacuoles. Desmin and Myotilin stainings also pointed to accumulation as in Myofibrillar Myopathy. This report further confirms that STM and LGMD2H represent the same disorder and suggests to consider TRIM32 mutations in the genetic diagnosis of Sarcotubular Myopathy and Myofibrillar Myopathy.
Keywords: LGMD2H; TRIM32; desmin; myotilin; sarcotubular myopathy; spheroids bodies.
Figures


References
-
- Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of ‘single protein RING finger’ E3 ubiquitin ligases. Bioessays 2005;27:1147-57. - PubMed
-
- Lazzari E, Meroni G. TRIM32 ubiquitin E3 ligase, one enzyme for several pathologies: from muscular dystrophy to tumours. Int J Biochem Cell Biol 2016;79:469-77. - PubMed
-
- Albor A, El-Hizawi S, Horn EJ, et al. The interaction of Piasy with TRIM32, an E3-ubiquitin ligase mutated in limb-girdle muscular dystrophy type 2H, promotes Piasy degradation and regulates UVB-induced keratinocyte apoptosis through NFkappaB. J Biol Chem 2006;281:25850-66. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
