Interventions for morphea
- PMID: 31309547
- PMCID: PMC6630193
- DOI: 10.1002/14651858.CD005027.pub5
Interventions for morphea
Abstract
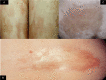
Background: Morphea (morphoea) is an immune-mediated disease in which excess synthesis and deposition of collagen in the skin and underlying connective tissues results in hardened cutaneous areas. Morphea has different clinical features according to the subtype and stage of evolution of the disease. There is currently no consensus on optimal interventions for morphea.
Objectives: To assess the effects of treatments for people with any form of morphea.
Search methods: We searched the following databases up to July 2018: the Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase, LILACS, and five trial registers. We checked the reference lists of included studies for further references to relevant randomised controlled trials.
Selection criteria: Randomised controlled trials of topical, intralesional, or systemic treatments (isolated or combined) in anyone who has been clinically diagnosed by a medical practitioner with any form of morphea. Eligible controls were placebo, no intervention, any other treatment, or different doses or duration of a treatment.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. The primary outcomes were global improvement of disease activity or damage assessed by a medical practitioner or by participants, and adverse effects. Secondary outcomes were improvement of disease activity and improvement of disease damage. We used GRADE to assess the quality of the evidence for each outcome.
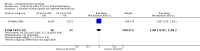
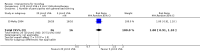
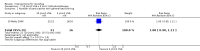
Main results: We included 14 trials, with a total of 429 randomised participants, aged between 3 and 76 years. There were juvenile and adult participants; over half were female, and the majority had circumscribed morphea, followed by linear scleroderma. The settings of the studies (where described) included a dermatologic centre, a national laboratory centre, paediatric rheumatology and dermatology centres, and a university hospital or medical centre.The studies evaluated heterogenous therapies for different types of morphea, covering a wide range of comparisons. We were unable to conduct any meta-analyses. Seven studies investigated topical medications, two evaluated intralesional medications, and five investigated systemic medications. The study duration ranged from seven weeks to 15 months from baseline.We present here results for our primary outcomes for our four key comparisons. All of these results are based on low-quality evidence.The included studies were at high risk of performance, detection, attrition, and reporting bias.Global improvement of disease activity or damage after treatment may be higher with oral methotrexate (15 mg/m², maximum 20 mg, once a week, for 12 months or until disease flare) plus oral prednisone (1 mg/kg a day, maximum of 50 mg, in a single morning dose, for three months, and one month with gradually decreased dose until discontinuation) than with placebo plus oral prednisone in children and adolescents with active morphea (linear scleroderma, generalised morphea or mixed morphea: linear and circumscribed) (risk ratio (RR) 2.31, 95% confidence interval (CI) 1.20 to 4.45; number needed to treat for an additional beneficial outcome (NNTB) 3; 1 randomised controlled trial (RCT); 70 participants, all juvenile). This outcome was measured 12 months from the start of treatment or until flare of the disease. Data were not available separately for each morphea type. There may be little or no difference in the number of participants experiencing at least one adverse event with oral methotrexate (26/46) or placebo (11/24) (RR 1.23, 95% CI 0.75 to 2.04; 1 RCT; 70 participants assessed during the 12-month follow-up). Adverse events related to methotrexate included alopecia, nausea, headache, fatigue and hepatotoxicity, whilst adverse events related to prednisone (given in both groups) included weight gain (more than 5% of body weight) and striae rubrae.One three-armed RCT compared the following treatments: medium-dose (50 J/cm²) UVA-1; low-dose (20 J/cm²) UVA-1; and narrowband UVB phototherapy. There may be little or no difference between treatments in global improvement of disease activity or damage, as assessed through the modified skin score (where high values represent a worse outcome): medium-dose UVA-1 phototherapy versus low-dose UVA-1 group: MD 1.60, 95% CI -1.70 to 4.90 (44 participants); narrowband UVB phototherapy versus medium-dose UVA-1 group: MD -1.70, 95% CI -5.27 to 1.87 (35 participants); and narrowband UVB versus low-dose UVA-1 group: MD -0.10, 95% CI -2.49 to 2.29 (45 participants). This RCT included children and adults with active morphea (circumscribed morphea, linear scleroderma (with trunk/limb variant and head variant), generalised morphea, or mixed morphea), who received phototherapy five times a week, for eight weeks. Outcomes were measured at eight weeks from the start of treatment.Safety data, measured throughout treatment, from the same RCT (62 participants) showed that treatment with UVA-1 phototherapy may cause mild tanning compared to narrowband UVB: narrowband UVB versus medium-dose UVA-1: RR 0.03, 95% CI 0.00 to 0.42; 35 participants; narrowband UVB versus low-dose UVA-1: RR 0.03, 95% CI 0.00 to 0.41; 45 participants. However, there may be no difference in the number of participants reporting mild tanning when comparing medium and low dose UVA-1 phototherapy (RR 1.00, 95% CI 0.91 to 1.10; 44 participants). Transient erythema was reported in three participants with narrowband UVB and no participants in the low- or medium-dose UVA-1 groups.
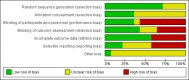
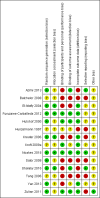
Authors' conclusions: Compared to placebo plus oral prednisone, oral methotrexate plus oral prednisone may improve disease activity or damage in juvenile active morphea (linear scleroderma, generalised morphea or mixed morphea: linear and circumscribed), but there may be a slightly increased chance of experiencing at least one adverse event.When medium-dose UVA-1 (50 J/cm²), low-dose UVA-1 (20 J/cm²), and narrowband UVB were compared against each other in treating children and adults with active morphea (circumscribed morphea, linear scleroderma, generalised morphea and mixed morphea), there may be little or no difference between these treatments on global improvement of disease activity or damage. UVA-1 phototherapy may cause more mild tanning than narrowband UVB, but there may be no difference between medium- and low-dose UVA-1 phototherapy. These results are based on low-quality evidence.Limitations of data and analyses include risk of bias and imprecision (small number of participants or events and wide confidence intervals). We encourage multicentre RCTs to increase sample size and evaluate, with validated tools, different treatment responses according to the subtypes of morphea and age groups.
Conflict of interest statement
Julia V de Albuquerque: nothing to declare. Brenda NG Andriolo: nothing to declare. Monica RA Vasconcellos: nothing to declare. Anne Lyddiatt: nothing to declare. Vinicius T Civile: nothing to declare. Virginia FM Trevisani: nothing to declare.
Figures














































































Update of
References
References to studies included in this review
Azimi 2013 {published data only}
-
- Azimi H, Golfroushan F, Nasimi M. Comparison of hydroxychloroquine and methotrexate in treatment of patients with localized scleroderma. Medical Journal of the Egyptian Armed Forces 2013;35(3):60‐5.
Batchelor 2008 {published data only}
-
- Batchelor R, Lamb S, Goulden V, Stables G, Goodfield M, Merchant W. Photodynamic therapy for the treatment of morphoea. Clinical and Experimental Dermatology 2008;33(5):661‐3. [CENTRAL: CN‐00666991; PUBMED: 18627394] - PubMed
El‐Mofty 2004 {published data only}
-
- El‐Mofty M, Mostafa W, El‐Darouty M, Bosseila M, Nada H, Yousef R, et al. Different low doses of broad‐band UVA in the treatment of morphea and systemic sclerosis. Photodermatology, Photoimmunology & Photomedicine 2004;20(3):148‐56. [CENTRAL: CN‐00516067; PUBMED: 15144393] - PubMed
Furuzawa‐Carballeda 2012 {published data only}
-
- Furuzawa‐Carballeda J, Ortiz‐Avalos M, Lima G, Jurado‐Santa Cruz F, Llorente L. Subcutaneous administration of polymerized type I collagen downregulates interleukin (IL)‐17A, IL‐22 and transforming growth factor‐beta1 expression, and increases Foxp3‐expressing cells in localized scleroderma. Clinical and Experimental Dermatology 2012;37(6):599‐609. [CENTRAL: CN‐00968871] - PubMed
Hulshof 2000 {published data only}
-
- Hulshof MM, Bouwes Bavinck JN, Bergman W, Masclee AA, Heickendorff L, Breedveld FC, et al. Double‐blind, placebo‐controlled study of oral calcitriol for the treatment of localized and systemic scleroderma. Journal of the American Academy of Dermatology 2000;43(6):1017‐23. [CENTRAL: CN‐00330232] - PubMed
Hunzelmann 1997 {published data only}
-
- Hunzelmann N, Anders S, Fierlbeck G, Hein R, Herrmann K, Albrecht M, et al. Double‐blind, placebo‐controlled study of intralesional interferon gamma for the treatment of localized scleroderma. Journal of the American Academy of Dermatology 1997;36(3 Pt 1):433‐5. [CENTRAL: CN‐00137759] - PubMed
Kreuter 2006 {published data only}
-
- Kreuter A, Hyun J, Stücker M, Sommer A, Altmeyer P, Gambichler T. A randomized controlled study of low‐dose UVA1, medium‐dose UVA1, and narrowband UVB phototherapy in the treatment of localized scleroderma. Journal of the American Academy of Dermatology 2006;54(3):440‐7. [CENTRAL: CN‐00555244] - PubMed
Kroft 2009a {published data only}
-
- Kroft EB, Groeneveld TJ, Seyger MM, Jong EM. Efficacy of topical tacrolimus 0.1 in active plaque morphea: Randomized, double‐blind, emollient‐controlled pilot study. American Journal of Clinical Dermatology 2009;10(3):181‐7. [CENTRAL: CN‐00699723] - PubMed
Noakes 2018 {published data only}
-
- Noakes R. Assessing the response of morphea and limited scleroderma to tranilast: a small prospective study comparing topical corticosteroids to a combination of topical corticosteroids and tranilast. Clinical, Cosmetic and Investigational Dermatology 2018;11:321‐26. [CENTRAL: CN‐01616903] - PMC - PubMed
Sator 2009 {published data only}
-
- Sator PG, Radakovic S, Schulmeister K, Honigsmann H, Tanew A. Medium‐dose is more effective than low‐dose ultraviolet A1 phototherapy for localized scleroderma as shown by 20‐MHz ultrasound assessment. Journal of the American Academy of Dermatology 2009;60(5):786‐91. [PUBMED: 19211170 ] - PubMed
Shalaby 2016 {published data only}
-
- Shalaby SM, Bosseila M, Fawzy MM, Abdel Halim DM, Sayed SS, Allam RS. Fractional carbon dioxide laser versus low‐dose UVA‐1 phototherapy for treatment of localized scleroderma: a clinical and immunohistochemical randomized controlled study. Lasers in Medical Science 2016;31(8):1707‐15. [CENTRAL: CN‐01263458] - PubMed
Tang 2006 {published data only}
-
- Tang Y, Treudler R, Tebbe B, Orfanos C. Treatment of localized scleroderma with herbs of traditional chinese medicine [Einsatz von Krautern der traditionellen chinesischen Medizin (TCM) bei der zirkumskripten Sklerodermie]. Kosmetische Medizin 2006;27(3):100‐9. [CENTRAL: CN‐00612744]
Yan 2013 {published data only}
-
- Yan XN, Zhang JR, Zhang CQ, Tian Q, Chen L, Chen L. Efficacy observation on acupuncture and moxibustion combined with hot compress of TCM herbs for scleroderma. Zhongguo Zhen Jiu [Chinese Acupuncture & Moxibustion] 2013;33(5):403‐6. [CENTRAL: CN‐00918725] - PubMed
Zulian 2011 {published data only}
-
- Zulian F, Martini G, Vallongo C, Vittadello F, Falcini F, Patrizi A, et al. Methotrexate treatment in juvenile localized scleroderma: a randomized, double‐blind, placebo‐controlled trial. Arthritis and Rheumatism 2011;63(7):1998‐2006. [CENTRAL: CN‐00801715] - PubMed
References to studies excluded from this review
Bodemer 1999 {published data only}
-
- Bodemer C, et al. Localized scleroderma in childhood and therapeutic trial with calcitriol: A therapeutic option to define [[French] Sclerodermies localisees de l'enfant et tentative therapeutique par calcitriol: Une possibilite therapeutique a definir]. Annales de Dermatologie et de Venereologie 1999;126(10):725‐726. - PubMed
Didenko 1978 {published data only}
-
- Didenko IG. Therapeutic effectiveness of ultrasonics and lidase phonophoresis in various forms of scleroderma. Vestnik Dermatologii i Venerologii 1978;6:76‐79. [CENTRAL: CN‐01131746] - PubMed
Dortu 1974 {published data only}
-
- Dortu J. Evaluation of Elarzone‐Dausse phlebology. Phlebologie 1974;27(3):381‐384. [PUBMED: 4614284] - PubMed
Dytoc 2014 {published data only}
-
- Dytoc M, Wat H, Cheung‐Lee M, Sawyer D, Ackerman T, Fiorillo L. Evaluation of the efficacy and safety of topical imiquimod 5% for plaque‐type morphea: a multicenter, prospective, vehicle‐controlled trial. Journal of Cutaneous Medicine & Surgery 2015;19(2):132‐139. [CENTRAL: CN‐01097258] - PubMed
Hu 1996 {published data only}
-
- Hu G, Zhu Y, Ran LH, Wang ZY. Clinical observation on the efficacy of Prostaglandin E1 and Propylene glycol alginate sodium in the treatment of scleroderma. Chinese journal of dermatology 1996;29(4):284‐285. [CENTRAL: CN‐00843757]
Smirnov 1998 {published data only}
-
- Smirnov A. Gonadotherapy as a treatment of patients with localized scleroderma. Journal of the European Academy of Dermatology & Venereology 7‐11 Ocober 1998;11(Suppl 2):S273.
Wang 2008 {published data only}
-
- UVA1 Light for Treatment of Scleroderma and Similar Conditions. clinicaltrials.gov/ct2/show/NCT00476801 (first received 22 May 2007).
References to studies awaiting assessment
NCT00812188 {unpublished data only}
-
- NCT00812188. A prospective, open label trial of high dose UVA‐1, 3x/week or medium dose UVA‐1, 3x/week vs. fluocinonide 0.05% cream treatment of morphea. clinicaltrials.gov/ct2/show/record/NCT00812188 (first received 18 December 2008).
NCT01799174 {unpublished data only}
-
- NCT01799174. Treatment study comparing UVA‐1 phototherapy versus placebo treatment for morphea. clinicaltrials.gov/ct2/show/NCT01799174 (first received 26 February 2013).
Additional references
Arkachaisri 2010
Asano 2018
-
- Asano Y, Fujimoto M, Ishikawa O, Sato S, Jinnin M, Takehara K, et al. Diagnostic criteria, severity classification and guidelines of localized scleroderma. Journal of Dermatology 2018;45(7):755‐80. - PubMed
Badea 2009
-
- Badea I, Taylor M, Rosemberg A, Foldvari M. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and systemic sclerosis. Rheumatology 2009;48(3):213‐21. [PUBMED: 19022832] - PubMed
Barnes 2012
-
- Barnes J, Mayer MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Current Opinion in Rheumatology 2012;24(2):165‐70. [PUBMED: 22269658] - PubMed
Beyer 2012
-
- Beyer C, Distler O, Distler JH. Innovative antifibrotic therapies in systemic sclerosis. Current Opinion in Rheumatology 2012;24(3):274‐80. [PUBMED: 22450392] - PubMed
Bielsa Marsol 2013
Careta 2015
Chen 2002
-
- Chen K, See A, Shumack S. Epidemiology and pathogenesis of scleroderma. Australasian Journal of Dermatology 2003;44(1):1‐9. [PUBMED: 12581091] - PubMed
Clements 1995
-
- Clements PH, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. Journal of Rheumatology 1995;22(7):1281‐5. [PUBMED: 7562759] - PubMed
CS‐COUSIN
-
- Cochrane Skin ‐ Core Outcome Set Initiative. cs‐cousin.org/ (accessed 04 March 2019).
Dytoc 2005
-
- Dytoc M, Ting PT, Man J, Sawyer D, Fiorillo L. First case series on the use of imiquimod for morphoea. British Journal of Dermatology 2005;153(4):815‐20. - PubMed
Fett 2011a
-
- Fett N, Werth VP. Update on morphea. Part I. Epidemiology, clinical presentation, and pathogenesis. Journal of the American Academy of Dermatology 2011;64(2):217‐28. [PUBMED: 21238823] - PubMed
Fett 2011b
Fett 2012
-
- Fett NM. Morphea: Evidence‐based recommendations for treatment. Indian Journal of Dermatology, Venereology and Leprology 2012;78(2):135‐41. [PUBMED: 22421642] - PubMed
Fett 2013
-
- Fett, N. Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies. Clinics in dermatology 2013;31.4:432‐437. - PubMed
GRADE Handbook
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 23 July 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guillevin 1983
-
- Guillevin L, Ortonne JP. Treatment of scleroderma [Traitement de la sclerodermie]. Annals of Internal Medicine 1983;134(8):754‐65. [PUBMED: 6364917] - PubMed
Harding 1998
Hawk 2001
-
- Hawk A, English JC. Localized and systemic scleroderma. Seminars in Cutaneous Medicine and Surgery 2001;20(1):27‐37. [PUBMED: 11308134] - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hunzelmann 1998
-
- Hunzelmann N, Scharffetter Kochanek K, Hager C, Krieg T. Management of localized scleroderma. Seminars in Cutaneous Medicine & Surgery March 1998;17(1):34‐40. [PUBMED: 9512105] - PubMed
Johnson 2012
-
- Johnson W, Jacobe H. Morphea in adults and children cohort II: Patients with morphea experience delay in diagnosis and large variation in treatment. Journal of the American Academy of Dermatology November 2012;67(5):881‐9. [PUBMED: 22382198] - PubMed
Kahaleh 1985
-
- Kahaleh MB, Sultany GL, Smith EA, Huffstutter JE, Loadholt CB, LeRoy EC. A modified scleroderma skin scoring method. Clinical and Experimental Rheumatology 1985;4(4):367‐9. - PubMed
Kahan 1989
-
- Kahan A, Amor B, Menkes CJ, Strauch G. Recombinant interferon‐γ in the treatment of systemic sclerosis. American Journal of Medicine 1989;87(3):273‐7. [PUBMED: 2505614] - PubMed
Knobler 2017
-
- Knobler R, Moinzadeh P, Hunzelmann N, Kreuter A, Cozzio A, Mouthon L, et al. European Dermatology Forum S1‐guideline on the diagnosis and treatment of sclerosing diseases of the skin, Part 1: localized scleroderma, systemic sclerosis and overlap syndromes. Journal of the European Academy of Dermatology and Venereology : JEADV 2017;31(9):1401‐24. - PubMed
Kreuter 2015
-
- Kreuter A, Hunzelmann N. Recurrence rates in localized scleroderma (morphoea). British Journal of Dermatology 2015;172(3):562‐3. [PUBMED: 25776242] - PubMed
Kroft 2009b
-
- Kroft EB, Jong EM, Evers AW II. Psychological distress in patients with morphea and eosinophilic fasciitis. Archives of Dermatology 2009;145(9):1017‐22. [PUBMED: 19770441] - PubMed
Laxer 2006
-
- Laxer RM, Zulian F. Localized scleroderma. Current Opinion in Rheumatology 2006;18(6):606‐13. [PUBMED: 17053506] - PubMed
Leheta 2013
-
- Leheta T, Garem Y, Hegazy R, Abdel Hay RM, Abdel Halim D. Non‐ablative 1540 fractional laser: how far could it help injection lipolysis and dermal fillers in lower‐face rejuvenation? A randomized controlled trial. Journal of Cosmetic and Laser Therapy 2013;15(1):13‐20. [PUBMED: 23057533] - PubMed
Leitenberger 2009
Li 2012
-
- Li SC, Torok KS, Pope E, Dedeoglu F, Hong S, Jacobe HT, et al. Development of consensus treatment plans for juvenile localized scleroderma: a roadmap toward comparative effectiveness studies in juvenile localized scleroderma. Arthritis Care & Research 2012;64(8):1175‐85. [PUBMED: 22505322] - PMC - PubMed
Marsol 2013
-
- Bielsa Marsol I. Update on the classification and treatment of localized scleroderma. Actas Dermo‐Sifiliográficas 2013;104(8):654‐66. [PUBMED: 23948159] - PubMed
Mayes 1998
-
- Mayes MD. Classification and epidemiology of scleroderma. Seminars in cutaneous medicine and surgery 1998;17(1):22‐26. - PubMed
Mertens 2015
-
- Mertens JS, Seyger MMB, Kievit W, Hoppenreijs EPAH, Jansen TL, Kerkhof PCM, et al. Disease recurrence in localized scleroderma: a retrospective analysis of 344 patients with paediatric‐or adult‐onset disease. British Journal of Dermatology 2015;172(3):722‐8. [PUBMED: 25381928 ] - PubMed
Peterson 1997
-
- Peterson LS, Nelson AM, Su WP, Mason T, O'Fallon W M, Gabriel SE. The epidemiology of morphea (localized scleroderma) in Olmsted County 1960‐1993. The Journal of rheumatology 1997;4(1):73‐80. - PubMed
Pope 1998a
Pope 1998b
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rook 2010
-
- Breathnach SM. 73. Drug Reactions. In: Burns T, Breathnach S, Cox N, Griffiths C editor(s). Rook's Textbook of Dermatology. 8th Edition. Vol. 4, Wiley‐Blackwell, 2010:73.1‐73.177.
Ruperto 2001
-
- Ruperto N, Ravelli A, Pistorio A, Malattia C, Viola S, Cavuto S, et al. Paediatric Rheumatology International Trials Organisation. The Italian version of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ). Clinical and Experimental Rheumatology 2001;19(4 Suppl 23):S91‐5. [PUBMED: 11510339] - PubMed
Saxton‐Daniels 2010
Schulz 2010
Steen 1982
-
- Steen VD, Medsger TA, Rodnan GP. D‐Penicillamine therapy in progressive systemic sclerosis (scleroderma). Annals of Internal Medicine 1982;97(5):652‐9. [PUBMED: 7137731] - PubMed
Tingey 1998
Tratenberg 2017
-
- Tratenberg M, Gutwein F, Rao V, Sperber K, Wasserrman A, Ash J. Localized scleroderma: a clinical review. Current Rheumatology Reviews 2017;13(2):86‐92. [PUBMED: 27604889] - PubMed
Valanciene 2010
-
- Valanciene G, Jasaitiene D, Valiukeviciene S. Pathogenesis and treatment modalities of localized scleroderma. Medicina (Kaunas, Lithuania) 2010;46(10):649‐56. [PUBMED: 21393982] - PubMed
Vasquez 2012
-
- Vasquez R, Sendejo C, Jacobe H. Morphea and other localized forms of scleroderma. Current Opinion in Rheumatology 2012;24(6):685‐93. [PUBMED: 23018858] - PubMed
Vilela 2010
-
- Vilela FA, Carneiro S, Ramos‐e‐Silva M. Treatment of morphea or localized scleroderma: review of the literature. Journal of Drugs in Dermatology 2010;9(10):1213‐19. [PUBMED: 20941945] - PubMed
Zachariae 1994
-
- Zachariae H, Bjerring P, Halkier Sorensen L, Heickendorff L, Sondergaard K. Skin scoring in systemic sclerosis: a modification—relations to subtypes and the aminoterminalpropeptide of type III procollagen (PIIINP). Acta Dermato‐venereologica 1994;74(6):444‐6. [PUBMED: 7701875] - PubMed
Zandi 2012
-
- Zandi S, Kalia S, Lui H. UVA1 phototherapy: a concise and practical review. Skin Therapy Letter 2012;17(1):1‐4. [PUBMED: 22358227] - PubMed
Zwischenberger 2011
-
- Zwischenberger BA, Jacobe HT. A systematic review of morphea treatments and therapeutic algorithm. Journal of the American Academy of Dermatology 2011;65(5):925‐41. [PUBMED: 21645943] - PubMed
References to other published versions of this review
Ravelli 2014
-
- Ravelli FN, Andriolo BNG, Vasconcellos MRA, Lyddiatt A, Fernandes Moça Trevisani V. Interventions for morphea. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD005027.pub4] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

