Glial and neuronal expression of the Inward Rectifying Potassium Channel Kir7.1 in the adult mouse brain
- PMID: 31309576
- PMCID: PMC6794205
- DOI: 10.1111/joa.13048
Glial and neuronal expression of the Inward Rectifying Potassium Channel Kir7.1 in the adult mouse brain
Abstract
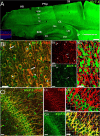
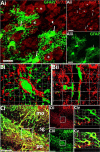
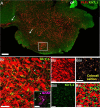
Inward Rectifying Potassium channels (Kir) are a large family of ion channels that play key roles in ion homeostasis and neuronal excitability. The most recently described Kir subtype is Kir7.1, which is known as a K+ transporting subtype. Earlier studies localised Kir7.1 to subpopulations of neurones in the brain. However, the pattern of Kir7.1 expression across the brain has not previously been examined. Here, we have determined neuronal and glial expression of Kir7.1 in the adult mouse brain, using immunohistochemistry and transgenic mouse lines expressing reporters specific for astrocytes [glial fibrillary acidic protein-enhanced green fluorescent protein (GFAP-EGFP], myelinating oligodendrocytes (PLP-DsRed), oligodendrocyte progenitor cells (OPC, Pdgfra-creERT2 /Rosa26-YFP double-transgenic mice) and all oligodendrocyte lineage cells (SOX10-EGFP). The results demonstrate significant neuronal Kir7.1 immunostaining in the cortex, hippocampus, cerebellum and pons, as well as the striatum and hypothalamus. In addition, astrocytes are shown to be immunopositive for Kir7.1 throughout grey and white matter, with dense immunostaining on cell somata, primary processes and perivascular end-feet. Immunostaining for Kir7.1 was observed in oligodendrocytes, myelin and OPCs throughout the brain, although immunostaining was heterogeneous. Neuronal and glial expression of Kir7.1 is confirmed using neurone-glial cortical cultures and optic nerve glial cultures. Notably, Kir7.1 have been shown to regulate the excitability of thalamic neurones and our results indicate this may be a widespread function of Kir7.1 in neurones throughout the brain. Moreover, based on the function of Kir7.1 in multiple transporting epithelia, Kir7.1 are likely to play an equivalent role in the primary glial function of K+ homeostasis. Our results indicate Kir7.1 are far more pervasive in the brain than previously recognised and have potential importance in regulating neuronal and glial function.
Keywords: Kir7.1; astrocytes; immunohistochemistry; neurones; oligodendrocytes.
© 2019 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Conflict of interest statement
A.B. is a shareholder and co‐founder of GliaGenesis Ltd.
Figures




Similar articles
-
A critical role for the inward rectifying potassium channel Kir7.1 in oligodendrocytes of the mouse optic nerve.Brain Struct Funct. 2020 Apr;225(3):925-934. doi: 10.1007/s00429-020-02043-4. Epub 2020 Feb 21. Brain Struct Funct. 2020. PMID: 32086565 Free PMC article.
-
Expression of Kir4.1 and Kir5.1 inwardly rectifying potassium channels in oligodendrocytes, the myelinating cells of the CNS.Brain Struct Funct. 2017 Jan;222(1):41-59. doi: 10.1007/s00429-016-1199-8. Epub 2016 Feb 15. Brain Struct Funct. 2017. PMID: 26879293 Free PMC article.
-
Inwardly rectifying potassium channels (Kir) in central nervous system glia: a special role for Kir4.1 in glial functions.J Cell Mol Med. 2006 Jan-Mar;10(1):33-44. doi: 10.1111/j.1582-4934.2006.tb00289.x. J Cell Mol Med. 2006. PMID: 16563220 Free PMC article. Review.
-
Progressive loss of a glial potassium channel (KCNJ10) in the spinal cord of the SOD1 (G93A) transgenic mouse model of amyotrophic lateral sclerosis.J Neurochem. 2006 Nov;99(3):900-12. doi: 10.1111/j.1471-4159.2006.04131.x. Epub 2006 Aug 21. J Neurochem. 2006. PMID: 16925593
-
The unique structural characteristics of the Kir 7.1 inward rectifier potassium channel: a novel player in energy homeostasis control.Am J Physiol Cell Physiol. 2023 Mar 1;324(3):C694-C706. doi: 10.1152/ajpcell.00335.2022. Epub 2023 Jan 30. Am J Physiol Cell Physiol. 2023. PMID: 36717105 Free PMC article. Review.
Cited by
-
Chronic Intermittent Ethanol Exposure Alters Behavioral Flexibility in Aged Rats Compared to Adult Rats and Modifies Protein and Protein Pathways Related to Alzheimer's Disease.ACS Omega. 2022 Dec 7;7(50):46260-46276. doi: 10.1021/acsomega.2c04528. eCollection 2022 Dec 20. ACS Omega. 2022. PMID: 36570296 Free PMC article.
-
Kir5.1 channels: potential role in epilepsy and seizure disorders.Am J Physiol Cell Physiol. 2022 Sep 1;323(3):C706-C717. doi: 10.1152/ajpcell.00235.2022. Epub 2022 Jul 18. Am J Physiol Cell Physiol. 2022. PMID: 35848616 Free PMC article. Review.
-
Transgenic models for investigating the nervous system: Currently available neurofluorescent reporters and potential neuronal markers.Biochim Biophys Acta Gen Subj. 2020 Jul;1864(7):129595. doi: 10.1016/j.bbagen.2020.129595. Epub 2020 Mar 12. Biochim Biophys Acta Gen Subj. 2020. PMID: 32173376 Free PMC article. Review.
-
A critical role for the inward rectifying potassium channel Kir7.1 in oligodendrocytes of the mouse optic nerve.Brain Struct Funct. 2020 Apr;225(3):925-934. doi: 10.1007/s00429-020-02043-4. Epub 2020 Feb 21. Brain Struct Funct. 2020. PMID: 32086565 Free PMC article.
-
Oligodendrocyte Progenitors in Glial Scar: A Bet on Remyelination.Cells. 2024 Jun 12;13(12):1024. doi: 10.3390/cells13121024. Cells. 2024. PMID: 38920654 Free PMC article. Review.
References
-
- Ango F, di Cristo G, Higashiyama H, et al. (2004) Ankyrin‐based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell 119, 257–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

