Reference-based sensitivity analysis for time-to-event data
- PMID: 31309730
- PMCID: PMC6899641
- DOI: 10.1002/pst.1954
Reference-based sensitivity analysis for time-to-event data
Abstract
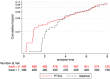
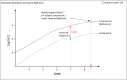
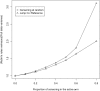
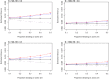
The analysis of time-to-event data typically makes the censoring at random assumption, ie, that-conditional on covariates in the model-the distribution of event times is the same, whether they are observed or unobserved (ie, right censored). When patients who remain in follow-up stay on their assigned treatment, then analysis under this assumption broadly addresses the de jure, or "while on treatment strategy" estimand. In such cases, we may well wish to explore the robustness of our inference to more pragmatic, de facto or "treatment policy strategy," assumptions about the behaviour of patients post-censoring. This is particularly the case when censoring occurs because patients change, or revert, to the usual (ie, reference) standard of care. Recent work has shown how such questions can be addressed for trials with continuous outcome data and longitudinal follow-up, using reference-based multiple imputation. For example, patients in the active arm may have their missing data imputed assuming they reverted to the control (ie, reference) intervention on withdrawal. Reference-based imputation has two advantages: (a) it avoids the user specifying numerous parameters describing the distribution of patients' postwithdrawal data and (b) it is, to a good approximation, information anchored, so that the proportion of information lost due to missing data under the primary analysis is held constant across the sensitivity analyses. In this article, we build on recent work in the survival context, proposing a class of reference-based assumptions appropriate for time-to-event data. We report a simulation study exploring the extent to which the multiple imputation estimator (using Rubin's variance formula) is information anchored in this setting and then illustrate the approach by reanalysing data from a randomized trial, which compared medical therapy with angioplasty for patients presenting with angina.
Keywords: MNAR; missing data; multiple imputation; sensitivity analysis; time to event.
© 2019 The Authors. Pharmaceutical Statistics published by John Wiley & Sons Ltd.
Figures
References
-
- United States National Research Council . The Prevention and Treatment of Missing Data in Clinical Trials, Panel on Handling Missing Data in Clinical Trials, Committee on National Statistics, Division of Behavioral and Social Sciences and Education Washington DC: The National Academic Press; 2010.
-
- CHMP . Guidelines on missing data in confirmatory clinical trials. European Medicines Agency, download from http://www.ema.europa.eu, accessed 15 January 2014.
-
- CHMP . Ich e9 (r1) addendum on estimands and sensitvity analysis in clinical trials to the guidance on statistical principles for clinical trials. European Medicines Agency, download from http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin...
-
- Scharfstein DO, Rotnitzky A, Robins JM. Adjusting for nonignorable drop‐out using semiparametric nonresponse models. J Am Stat Assoc. 1999;94(448):1096‐1120.
-
- Scharfstein DO, Robins JM, Eddings W, Rotnitzky A. Inference in randomized studies with informative censoring and discrete time‐to‐event endpoints. Biometrics. 2001;57:404‐413. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

