Rift Valley fever: biology and epidemiology
- PMID: 31310198
- PMCID: PMC7613496
- DOI: 10.1099/jgv.0.001296
Rift Valley fever: biology and epidemiology
Abstract
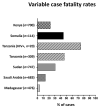
Rift Valley fever (RVF) is a mosquito-borne viral zoonosis that was first discovered in Kenya in 1930 and is now endemic throughout multiple African countries and the Arabian Peninsula. RVF virus primarily infects domestic livestock (sheep, goats, cattle) causing high rates of neonatal mortality and abortion, with human infection resulting in a wide variety of clinical outcomes, ranging from self-limiting febrile illness to life-threatening haemorrhagic diatheses, and miscarriage in pregnant women. Since its discovery, RVF has caused many outbreaks in Africa and the Arabian Peninsula with major impacts on human and animal health. However, options for the control of RVF outbreaks are limited by the lack of licensed human vaccines or therapeutics. For this reason, RVF is prioritized by the World Health Organization for urgent research and development of countermeasures for the prevention and control of future outbreaks. In this review, we highlight the current understanding of RVF, including its epidemiology, pathogenesis, clinical manifestations and status of vaccine development.
Keywords: Rift Valley fever; epidemiology; one health; pathogenesis; transmission; vaccine.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



References
-
- Daubney R, Hudson JR, Garnham PC. Enzootic hepatitis or Rift Valley fever. an undescribed virus disease of sheep cattle and man from East Africa. J Pathol Bacteriol. 1931;34:545–579.
-
- Daubney R, Hudson JR. Rift Valley fever. East African Medical Journal. 1933;10:2–19.
-
- Gear J, De Meillon B, Le Roux AF, Kofsky R, Innes RR, et al. Rift Valley fever in South Africa; a study of the 1953 outbreak in the orange free state, with special reference to the vectors and possible reservoir hosts. S Afr Med J. 1955;29:514–518. - PubMed
-
- Easterday BC. Rift Valley fever. Adv Vet Sci. 1965;10:65–127. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

